3640
The roles of sensorimotor and default mode-memory retrieval networks in staging PD cognitive decline1Department of Diagnostic Radiology, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Kaohsiung, Taiwan, 2Department of Biomedical Engineering and Biomedical Research Imaging Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3Department of Radiology and Biomedical Research Imaging Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Synopsis
In addition to motor disability, the development of cognitive decline is one of the most notorious symptoms in Parkinson’s disease (PD). Yet, a biomarker capable of accurately diagnosing cognitive impairments in PD patients remains elusive. Our results, based on resting-state functional MRI, suggested that functional connectivity in sensorimotor together with default mode-memory retrieval networks may serve a promising biomarker either for staging cognitive decline or neuropsychiatric scores.
Introduction:
Patients with PD often suffer from both motor and non-motor symptoms simultaneously. In particular, the development of dementia is the most notorious symptom that occurs in nearly 80% prevalence rate in PD patients (1). While most of the studies aiming to shed light on the progression of cognitive impairment in PD have largely focused on the relations between the default mode network and/or memory and cognitive impairment(2, 3), the potential interplay between motor symptoms and cognitive impairment in PD patients remains elusive, particularly in the lack of dopaminergic effects (4). It is plausible that parts of motor symptoms may arise from impairment of sensorimotor integration caused by cognitive deficits (5, 6). To this end, evaluation of functional connectivity alteration in both motor and cognitive-associated networks among PD patients with normal cognition (PDN), mild cognitive impairment (PDMCI), and dementia (PDD) could reveal critical biological underpinnings leading to cognitive impairments in PD patients. Thus, this study aimed to assess the patterns and the neuropsychiatric associations of functional MRI, including both the motor and cognitive-associated networks in patients across the three stages (PDN, PDMCI, and PDD) of cognitive decline in Parkinson Disease.Methods:
A total of 126 (65 males and 61 females) patients with idiopathic PD diagnosed according to the United Kingdom Brain Bank criteria (7) were prospectively enrolled at the Neurology Department, Kaohsiung Chang Gung Memorial Hospital. All patients received the Wechsler Adult Intelligence scale-III (WAIS-III) and the Cognitive Ability Screen Instrument (CASI) as the neuropsychiatric survey (8). Patients were grouped into 3 subgroups, including PDN, PDMCI, and PDD, according to the neuropsychiatric results by the Movement Disorder Society PD-MCI Task Force diagnostic criteria (9). Additionally, 50 age- and gender-matched normal controls (NC) were also enrolled. rsfMRI images were acquired before taking daily medication and processed using FSL(10-12), including discarding the first 10 volumes, slice-timing correction, motion correction, spatial smoothing (6mm FWHM), bandpass filtering (0.01 Hz ~ 0.08 Hz), global mean/white matter/CSF signal regression, as well as FD-DVARS (13) processing. After co-registering fMRI data of all subjects to a standard template, the Power brain function area parcellation (14) was used to define the default mode (DMN) -memory retrieval (MRN) and sensorimotor network (SMN) ROIs. T-test with FDR correction was performed determining different patterns of functional connectivity between the NC group and each of the three PD subgroups. ANCOVA after controlling age and gender was used for group-wised comparisons. Partial correlations were performed for the association between functional connectivity and neuropsychiatric results after controlling age and gender factors.
Results:
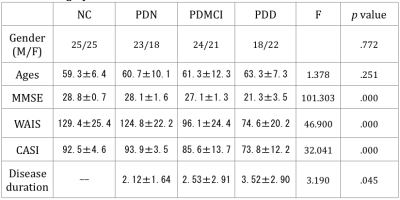
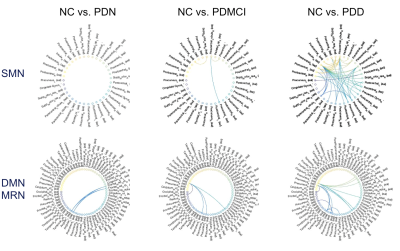
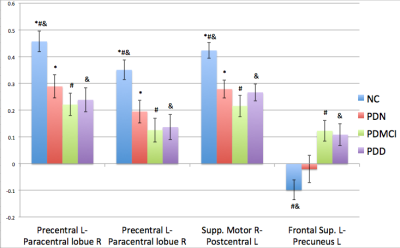
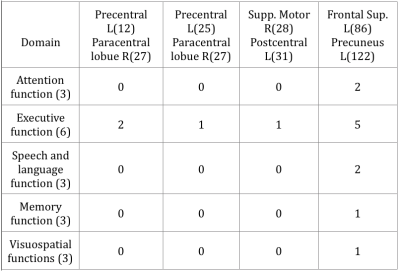
The demographic data was listed in table 1. The T-test for deficit pattern was showed in Figure 1. Four FC deficits are observed in both the PDMCI and PDD but not in PDN groups. Figure 2 showed the ANCOVA results for the 4 FC. For the partial correlations between FC and neuropsychiatric tests, several associations were summarized in tables 2. Of note, associations was observed between the FC of left superior frontal gyrus (SFG) and left precuneus and all of the functional domains, especially in executive function.Discussion:
Our findings suggest that the FC of the DMN-MRN and SMN could serve as potential biomarkers to differentiate cognitive impairment in PDMCI and PDD, from PDN. Although previously studies by others have emphasized the role of DMN-MRN in the demanded PD patients, our results clearly demonstrate the potential contributions of SM abnormalities to cognitive impairments in PD patients. Specifically, the FC of SMN are more extensively affected than DMN-MRN in both PDMCI (3) and PDD (15). In addition, an anticorrelation between the SFG and precuneus is observed in NC (Fig. 2) whereas the same connection becomes positively correlated in PDMCI and PDD. Furthermore, it is inversely associated with most subtests of WAIS and several of cognitive domains of the CASI. Although more studies are needed to further understand how interaction between these two regions are altered from NC to PDN, PDMCI and PDD patients, our results suggest that lost of this anticorrelation between the SFG and precuneus might aggravate the PD patient cognitive deficits.Conclusion:
Not only the DMN-MRN could affect the cognitive function, the SMN may play an important role in exacerbating the impaired cognitive performance in PD. With the high association between neuropsychiatric results and the FC, the combine usage of fMRI focusing on the DMN-MRN and the SMN would be a favorable biomarker for enhancing the cognitive impairment diagnostic accuracy.Acknowledgements
This work was supported by funds from the National Sci- ence Council (MOST 103-2314-B-182A-010-MY3 to Wei- Che Lin and MOST 104-2314-B-182A-053 to Hsiu-Ling Chen) and Chang Gung Memorial Hospital (CMRPG891511 and CMRPG8B0831 to Wei-Che Lin, CMRPG890801 and CMRPG8C0021 to Hsiu-Ling Chen, and CMRPG8E0621 to Meng-Hsiang Chen).References
1. Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387-92.
2. Ibarretxe-Bilbao N, Zarei M, Junque C, et al. Dysfunctions of cerebral networks precede recognition memory deficits in early Parkinson's disease. Neuroimage. 2011;57(2):589-97.
3. Hou Y, Yang J, Luo C, et al. Dysfunction of the Default Mode Network in Drug-Naive Parkinson's Disease with Mild Cognitive Impairments: A Resting-State fMRI Study. Front Aging Neurosci. 2016;8:247.
4. Tinaz S, Lauro PM, Ghosh P, Lungu C, Horovitz SG. Changes in functional organization and white matter integrity in the connectome in Parkinson's disease. Neuroimage Clin. 2017;13:395-404.
5. Georgiou N, Iansek R, Bradshaw JL, Phillips JG, Mattingley JB, Bradshaw JA. An evaluation of the role of internal cues in the pathogenesis of parkinsonian hypokinesia. Brain. 1993;116 ( Pt 6):1575-87.
6. Baumer T, Pramstaller PP, Siebner HR, et al. Sensorimotor integration is abnormal in asymptomatic Parkin mutation carriers: a TMS study. Neurology. 2007;69(21):1976-81.
7. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745-52.
8. Chen YS, Chen MH, Lu CH, et al. Associations among Cognitive Functions, Plasma DNA, and White Matter Integrity in Patients with Early-Onset Parkinson's Disease. Front Neurosci. 2017;11:9.
9. Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27(3):349-56.
10. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782-90.
11. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208-19.
12. Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1 Suppl):S173-86.
13. Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320-41.
14. Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72(4):665-78.
15. Rektorova I, Krajcovicova L, Marecek R, Mikl M. Default mode network and extrastriate visual resting state network in patients with Parkinson's disease dementia. Neurodegener Dis. 2012;10(1-4):232-7.
16. Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103(26):10046-51.
Figures