3630
Contribution of basal forebrain damage to cognitive deficits in Parkinson’s disease1Institut du Cerveau et de la Moelle épinière – ICM, Centre de NeuroImagerie de Recherche – CENIR, Paris, France, 2Sorbonne Universités, UPMC Univ Paris 06, Inserm U1127, CNRS UMR 7225, Paris, France, 3ICM Team Control of Normal and Abnormal Movement, Paris, France, 4Service de neuroradiologie, Groupe Hospitalier Pitié-Salpêtrière, AP-HP, Paris, France, 5Service de Neurologie, Hôpital Saint Anne, Paris, France, 6Département de Neurologie, Groupe Hospitalier Pitié-Salpêtrière, AP-HP, Paris, France
Synopsis
We investigated the contribution of basal forebrain damage in the cognitive dysfunction of 52 non-demented patients with Parkinson’s disease (PD) and 25 age-matched healthy controls using diffusion and resting state functional MRI. Patients showed diffusion changes in the basal forebrain and the fornix. They also showed reduced functional connectivity between the septal area and the temporal lobe including the hippocampi and parahippocampal gyri, and between the basal nucleus of Meynert and frontal areas and bilateral thalami. Structural and functional changes correlated with memory and executive functions.
Introduction
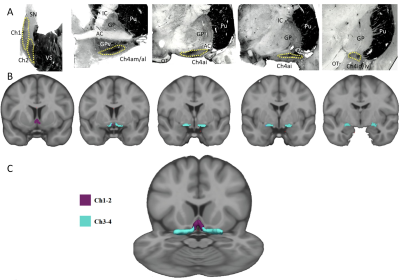
Cognitive deficits are frequently observed in patients with Parkinson’s disease (PD). In PD, histological studies have reported neuronal loss, presence of Lewy bodies and alpha-synuclein in the basal forebrain that were greater in PD patients with dementia as compared with non-demented PD (1,2). Using PET tracer of acetylcholinesterase activity, cortical cholinergic deficit was observed in PD with dementia (3,4). Here, we studied the relationships between damage of basal forebrain cholinergic structures and cognitive deficits presented by PD patients. We specifically investigated damage of different subdivisions of the basal forebrain, including the medial septal nucleus (Ch1), the nucleus of the vertical limb of the diagonal band (Ch2), the nucleus of the horizontal limb of the diagonal band (Ch3), and the nucleus basalis of Meynert (NBM or Ch4). These nuclei have different connections with the cortex, and contribute probably differently to the cognitive deficits. Ch1 and Ch2 project to the hippocampus, Ch3 to the olfactory bulb, and Ch4 to associative corticalregions and the amygdala (5).
Methods
Fifty-two PD patients with mild to moderate severity (mean age = 60.6±8.8 years, mean disease duration = 8.7±3.5 years, mean Hoehn and Yahr = 1.88±0.63) and 25 age- and gender-matched healthy volunteers (HV, mean age = 59.8±8.0 years) were recruited in this study. We investigated the relationship between changes in the different nuclei of the basal forebrain and the type of cognitive deficits in non-demented PD patients using tests of global cognitive (MMSE, Mattis dementia rating scale), memory (free and cued selective reminding test), visuospatial (Rey’s figure), and executive functions (TMT, stroop). We used diffusion MRI (dMRI) to quantify structural changes in the different nuclei of the basal forebrain from Ch1 to Ch4. We used diffusion-based tractography and resting state functional MRI (rsfMRI) to quantify changes in anatomical and functional connectivity respectively between Ch1-2 and the hippocampus via the fornix and between Ch3-4 and the cortex. MRI segmentation of Ch1 to Ch4 regions was based on histological sections that were obtained in the frame of previous histological study (6). We segmented Ch1 and Ch2 together, and Ch3 and Ch4 together because they were difficult to delineate one from another (5) (Figure1). dMRI analysis was performed using FSL software. rsfMRI analysis was performed using SPM8 and normalized masks of Ch1-2 and Ch3-4 that were taken as seed ROIs. A one-way ANCOVA was conducted to compare diffusion variables (FA, MD, AD and RD) inside the fornix, Ch1-2 and Ch3-4, between the groups (HV, PD). For rsfMRI, we ran a one-way ANOVA with 2 levels (PD, HV) to evaluate the difference between groups. Clusters were considered significant at p<0.05 corrected for multiple comparisons using family wise error and with a minimum cluster size at 20 voxels (height threshold at p <0.001 uncorrected). All statistics were performed by using the following covariates: age, gender, education, scores at the MADRS and Epworth scales.
Results
Diffusion data analysis
In PD patients, MD (HV=0.80±0.08, PD=0.85±0.08, p=0.029), AD (0.92±0.09 vs 0.98±0.08, p=0.029) and RD (0.74±0.08 vs 0.79±0.08, p=0.022) in Ch1-2. MD (0.74±0.09 vs 0.81±0.08, p=0.034) and RD (0.68±0.08 vs 0.73±0.08, p=0.034) increased in Ch3-4 and there was a trend for AD. In the fornix, only AD (1.15±0.09 vs 1.21±0.11, p=0.040) was increased and there was a trend for MD.
Structural connectivity
Ch1-2 showed no significant changes in the probability of connection density with the hippocampus. Ch3-4 showed significant decrease in the probability of connection density with the associative prefrontal cortex (HV: 117.0±286.6 PD: 20.8±28.5, p=0.023), occipital cortex (853.9±1247.8 vs 388.2±485.3, p=0.027) and peri-insular regions (548.2±959.9 vs 156.1±166.7, p=0.016).
Functional connectivity:
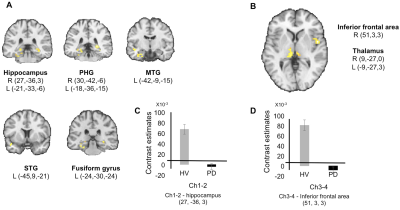
PD patients showed reduced functional connectivity between Ch1-2 and bilateral hippocampi and parahippocampal gyri, the left fusiform gyrus and the left superior and middle temporal gyri (Figure 2). PD patients showed reduced functional connectivity between Ch3-4 and the right inferior frontal area and bilateral thalamus (Figure 2).
Correlations with cognitive performances
In Ch1-2, loss of structural integrity and connectivity correlated with scores at the memory tests and the copy of Rey’s figure. Changes in Ch3-4 correlated with scores of global cognition and executive functions.
Conclusion
PD patients presented evidence of structural changes and abnormal structural and functional connectivity in the basal forebrain. Cognitive deficits associated with basal forebrain damage differed between Ch1-2 – hippocampal network (memory tests and the copy of Rey’s figure) and Ch3-4 – cortical network (global cognition and executive functions). These results confirm that basal forebrain regions are affected in PD and further suggest that they contribute differently to the cognitive impairment in PD. Further longitudinal study will determine whether the extent of structural and functional alterations in the basal forebrain cholinergic nuclei is associated with increased risk of dementia in PD.Acknowledgements
This work was supported by grants from ANR Nucleipark, DHOS-Inserm, France Parkinson, Ecole des NeuroSciences de Paris (ENP), Fondation pour la Recherche Médicale (FRM), and the Investissements d'Avenir, IAIHU-06 (Paris Institute of Neurosciences – IHU), ANR-11-INBS-0006, Fondation d’Entreprise EDF, and the Fondation Thérèse and René Planiol pour l’étude du Cerveau. We thank E.C. Hirsch, C. Duyckaerts, and Y. Agid for providing the histological material.References
1. Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003 Apr;24(2):197–211.
2. Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, et al. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain J Neurol. 2014 Sep;137(Pt 9):2493–508.
3. Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. 2003 Dec;60(12):1745–8.
4. Bohnen NI, Albin RL, Müller MLTM, Petrou M, Kotagal V, Koeppe RA, et al. Frequency of cholinergic and caudate nucleus dopaminergic deficits across the predemented cognitive spectrum of Parkinson disease and evidence of interaction effects. JAMA Neurol. 2015 Feb;72(2):194–200.
5. Mesulam M-M. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. J Comp Neurol. 2013 Dec 15;521(18):4124–44.
6. Lehéricy S, Hirsch ÉC, Cervera-Piérot P, Hersh LB, Bakchine S, Piette F, et al. Heterogeneity and selectivity of the degeneration of cholinergic neurons in the basal forebrain of patients with Alzheimer’s disease. J Comp Neurol. 1993 Apr 1;330(1):15–31.
Figures