3628
White Matter Alterations in Parkinson’s Disease Analyzed with Combined Diffusion Magnetic Resonance Imaging and Magnetization Transfer Saturation Imaging: A Tract-Based Spatial Statistics AnalysisChristina Andica1, Koji Kamagata1, Taku Hatano2, Asami Saito1, Yuki Takenaka1,3, Akifumi Hagiwara1,4, Masaaki Hori1, Ryusuke Irie1,4, Akihiko Wada1, Kanako Kumamaru1, Nobutaka Hattori2, and Shigeki Aoki1
1Department of Radiology, Juntendo University Graduate School of Medicine, Tokyo, Japan, 2Department of Neurology, Juntendo University School of Medicine, Tokyo, Japan, 3Department of Radiological Sciences, Graduate School of Human Health Sciences, Tokyo Metropolitan University, Tokyo, Japan, 4Department of Radiology, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
Synopsis
Our study combined three in-vivo imaging modalities (diffusion tensor imaging, neurite orientation dispersion and density imaging, and magnetization transfer saturation imaging) to provide a deeper understanding of white matter (WM) pathologies in Parkinson`s disease (PD). The tract-based spatial statistics analysis showed significantly decreased fractional anisotropy and intracellular volume fraction, and increased mean diffusivity, axial diffusivity, and radial diffusivity in an extensive area of WM in PD patients. Meanwhile, non-significantly increased myelin volume fraction was observed in limited areas of WM. The findings of this study might indicate that PD predominantly affects axons rather than myelin in WM.
Purpose
Parkinson`s disease (PD) is the second most common neurodegenerative disorder with classic motor symptoms, including tremor, rigidity, bradykinesia, and postural and gait impairment, and non-motor symptoms, including cognitive impairment, mood and sleep disorders, and autonomic dysfunction.1 PD is primarily considered as a gray matter (GM) disease, but recent histopathological studies indicate the involvement of white matter (WM) in the course of the disease, characterized by the accumulation of Lewy neurites in brainstem axons and later, in the cerebral WM.2 Although, the specific pathological changes in the WM of PD still remain unclear, mainly because brain autopsy studies confronted some limitations in the evaluation of WM.3 On the other hand, in-vivo MR imaging has shown its capability to capture microstructural changes, such as the alterations of axon and myelin.4 However, imaging findings on WM in PD have been inconsistent.5 Prior studies in PD have mainly focused either on diffusion-based imaging or myelin imaging, and predominantly used region-of-interest (ROI) methods.6-8 In the current study, we aimed to identify specific WM microstructural alterations in PD using a combination of diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI) as diffusion-based imaging, and magnetization transfer saturation (MT-sat) imaging as myelin imaging, analyzed by tract-based spatial statistics (TBSS).Methods
This study included 53 patients with PD and 38 age- and gender-matched healthy controls with the characteristics listed in Table 1. Patients and controls gave their informed consent to participate in this study, which was approved by the local Ethics Committee. All subjects were scanned on a 3 T MR scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with a 64-channel head coil. Each subject underwent multi-shell diffusion MRI consisting of two b values (1,000, and 2,000 s/mm2) acquired along 64 isotropic diffusion gradient directions and MT-sat imaging. TBSS9 was used to compare DTI indices (FA, fractional anisotropy; MD, mean diffusivity; AD, axial diffusivity; and RD, radial diffusivity), NODDI indices (ICVF, intracellular volume fraction; OD, orientation dispersion index; Viso, isotropic volume fraction), and MT-sat metric (MVF, myelin volume fraction) between PD and healthy control groups. Further, we also used TBSS to perform correlation analysis on all MR indices and clinical scores (disease duration and motor symptom severity assessed with UPDRS-III). A family-wise error corrected P-value of < 0.05 was considered significant for TBSS.Results
TBSS analysis showed significantly decreased FA and ICVF, and increased AD, RD, and MD in many major tracts of patients with PD compared with healthy controls (Fig. 1, Table 2). Meanwhile, non-significantly increased (P < 0.10) MVF was found in corticospinal tract, forceps minor, genu of corpus callosum, medial lemniscus, and corona radiata of patients with PD (Fig. 2). No correlations have been detected between all MR indices and disease duration or motor symptom severity assessed with UPDRS-III.Discussion
DTI has been widely used to investigate pathological changes in the WM of patients with various neurodegenerative diseases, including PD.3, 5 However, DTI-derived indices have been shown to be sensitive but not specific to microstructural changes.10 Decreased FA accompanied by increased MD, AD, and RD might reflect degeneration of WM microstructure due to axonal degeneration and demyelination,10 as shown in this study. On the contrary, NODDI enables more specific characterization of tissue microstructure by estimating ICVF and OD, the two key contributors of FA.10 In this study, patients with PD showed decreased ICVF, which reflects decreased neurite density, in almost the same area as did FA. Meanwhile, MT-sat imaging, which has been shown to be more specific to myelin content compared to diffusion-weighted imaging,11 showed non-significant increased MVF in some parts of the anterior-basal area of the brains of patients with PD. This finding is in line with a previous study that showed increased MVF in a group of PD patients with shorter disease duration.6 Some evidence has suggested that the WM microstructure in PD undergo compensatory or neuroplastic changes during the progression of PD and compensatory changes may be related to increases in myelin.6 Inferred from the results in our study, namely, decreased FA and Vic, increased AD, RD, and MD, and non-significantly increased MVF, we conclude that WM alterations in PD are predominantly caused by axonal damage and not by demyelination.Conclusion
DTI, NODDI, and MT-sat imaging can be used complementary to show WM pathologies in PD more specifically. The results of our study suggest that axonal degeneration is more predominant than demyelination in the WM microstructural alterations in PD.Acknowledgements
All authors have no conflict of interest to disclose.References
- Beitz JM. Parkinson's disease: a review. Front Biosci (Schol Ed) 2014;6:65-74
- Braak H, Ghebremedhin E, Rub U, et al. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res 2004;318:121-34
- Hattori T, Orimo S, Aoki S, et al. Cognitive status correlates with white matter alteration in Parkinson's disease. Hum Brain Mapp 2012;33:727-39
- Aung WY, Mar S, Benzinger TL. Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging Med 2013;5:427-40 5
- Wen MC, Heng HS, Ng SY, et al. White matter microstructural characteristics in newly diagnosed Parkinson's disease: An unbiased whole-brain study. Sci Rep 2016;6:35601
- Dean DC, 3rd, Sojkova J, Hurley S, et al. Alterations of Myelin Content in Parkinson's Disease: A Cross-Sectional Neuroimaging Study. PLoS One 2016;11:e0163774
- Kamagata K, Motoi Y, Tomiyama H, et al. Relationship between cognitive impairment and white-matter alteration in Parkinson's disease with dementia: tract-based spatial statistics and tract-specific analysis. Eur Radiol 2013;23:1946-55
- Surova Y, Lampinen B, Nilsson M, et al. Alterations of Diffusion Kurtosis and Neurite Density Measures in Deep Grey Matter and White Matter in Parkinson's Disease. PLoS One 2016;11:e0157755
- Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487-505
- Timmers I, Roebroeck A, Bastiani M, et al. Assessing Microstructural Substrates of White Matter Abnormalities: A Comparative Study Using DTI and NODDI. PLoS One 2016;11:e0167884
- Geeraert BL, Lebel RM, Mah AC, et al. A comparison of inhomogeneous magnetization transfer, myelin volume fraction, and diffusion tensor imaging measures in healthy children. Neuroimage 2017
Figures
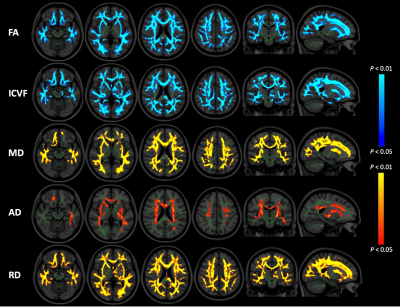
Figure 1. Comparison of FA, ICVF, MD, AD, and RD between patients with PD and healthy controls. TBSS analysis showed significantly (FWE corrected P < 0.05) decreased FA and ICVF (blue-light blue voxels) and significantly increased MD, AD, and RD (red-yellow voxels) in patients with PD when compared with healthy controls. The FA skeleton with FA > 0.2 is shown in green. To aid visualization, the results are thickened using the fill script implemented in FSL. AD, axial diffusivity; FA, fractional anisotropy; FWE, family-wise error; ICVF, intracellular volume fraction; MD, mean diffusivity; RD; radial diffusivity.
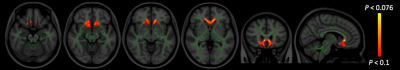
Figure 2. Comparison of MVF between patients with PD and healthy controls. TBSS analysis showed non-significantly increased (FWE corrected P < 0.10) MVF (red-yellow voxels) in patients with PD when compared with healthy controls. The FA skeleton with FA > 0.2 is shown in green. To aid visualization, the results are thickened using the fill script implemented in FSL. FA, fractional anisotropy; FWE, family-wise error; MVF, myelin volume fraction.
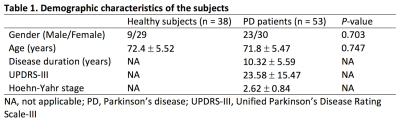
Table 1.
Demographic characteristics of the subjects.
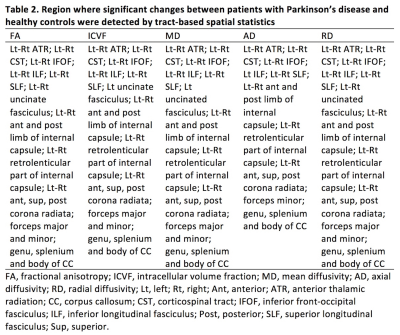
Table 2. Region where significant changes between patients with Parkinson’s disease and healthy controls were detected by tract-based spatial statistics.