3627
Automated White Matter Lesion Quantification Correlates With Gait and Cognitive Dysfunction In Parkinson’s Disease1Singapore General Hospital, Singapore, Singapore, 2Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 3Department of Radiology, Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 4Signal Processing Laboratory (LTS 5), École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 5National Neuroscience Institute, Singapore, Singapore, 6Nanyang Technological University, Singapore, Singapore, 7Siemens Healthcare, Singapore, Singapore, 8Duke-NUS Medical School, Singapore, Singapore
Synopsis
White matter lesions (WMLs) have an impact on neuronal connectivity; and consequently affect balance, mobility and cognition in both normal aging and disease states. Using a fully automated segmentation algorithm and multi-modal images, we estimated WMLs volumes to predict the clinical severity in a cohort of Parkinson’s disease (PD) patients and healthy controls (HC). Increased WMLs volume is strongly associated with both motor/gait and cognitive dysfunctions in PD. Lobar WMLs are found to have differential impact on distinctive cognitive domains. Automated volumetric quantification of WMLs load, particularly within the frontal and prefrontal regions can predict severity of symptoms in PD.
Introduction
White matter lesions (WMLs) or leukoaraiosis commonly presents as T1W-hypointensities/ T2W-hyperintensities on MRI. WMLs can affect balance, mobility and cognition in otherwise healthy old adults1, 2. Current literature on Parkinson’s Disease (PD) suggests that WMLs predominantly affect postural stability and gait motor functions3, 4. WMLs may cause these symptoms in PD by disrupting i) corticostriatal–thalamocortical loops5, ii) interhemispheric connections of the corpus callosum6 (critical for complex integrated motor programs), or iii) important sub-cortical afferents7.
Some studies8 attempted to evaluate the impact of WMLs (quantified using semi-automated tools) on cognition8, 9 in PD. Few studies10 have attempted to use multi-modal algorithms to improve the accuracy of WMLs detection. Here, we investigated the relationship of disease severity in a PD cohort with the WMLs burden estimated by a fully automated segmentation algorithm. We also studied the impact of WMLs distribution in specific brain regions with respect to clinical scores.
Methods
Whole-brain MR imaging (MRI) was performed at 1.5 and 3 Tesla (MAGNETOM Avanto; MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) on a case-control cohort of 41 subjects (16 PD and 25 age- and gender-matched HC). Two controls were excluded after post-screening due to cognitive dysfunction. The MRI protocol included: MPRAGE (TR/TI 2200/900ms; 230mm FOV; 256x256 matrix; 0.9mm slice thickness; 192 slices) and 3D FLAIR (TR/TE 5000/384ms; 230mm FOV; 256x256 matrix; 0.9 slice thickness; 192 slices) sequences.
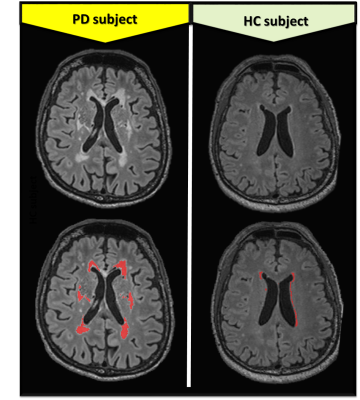
Brain regions and brain tissue segmentations were obtained automatically using the MorphoBox11 prototype on MPRAGE images. WMLs segmentations were assessed using an automated method based on a supervised approach12, 13, and refined using a partial volume estimation algorithm14, on MPRAGE and 3D FLAIR images. Using ITK-SNAP, quality of segmentation masks (see Figure 1) were reviewed by two of the authors trained in neuroanatomical landmarks with accompanying clinical neuroradiological reports.
WMLs volumes were estimated in six different brain regions to evaluate their relationship between motor/gait assessments and neuropsychological measures of executive and memory function. WMLs volumes were expressed as a percentage of total brain volume. Statistical analyses were performed using the following tests: i) bivariate correlation between WMLs and clinical scores using Pearson correlation; ii) repeated-measures ANOVA and a split-plot ANOVA to test for the predominant WMLs location; iii) stepwise regression with the entry criteria set at p ≤.05 and the remove criteria set at p ≥.10; iv) between-model regression coefficients differences using z-test15.
Results
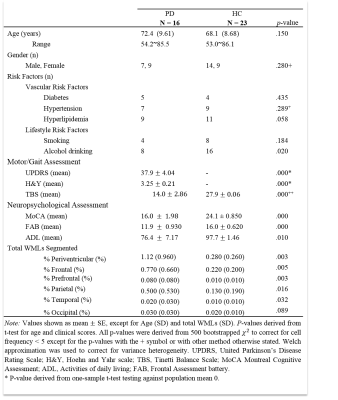
Table 1 shows summarized subject demographics, clinical characteristics and WMLs volumes. The groups are matched for vascular and lifestyle risk factors. Tinetti Balance Scale (TBS), Montreal Cognitive Assessment (MoCA) and Frontal Assessment battery (FAB) were significantly higher in PD (p< .001) compared to HC.
WMLs in total, frontal, prefrontal, parietal and periventricular regions were significantly higher in PD (p<.01) and negatively correlated (p<.05) with both MoCA and FAB. Temporal WMLs volumes were also significantly higher in PD (p<.01), but only correlated negatively (p<.05) with MoCA. Frontal and prefrontal WMLs negatively correlated with UPDRS and TBS (p<.05), but periventricular WMLs only correlated with worse UPDRS (p<.05) scores. Activities of daily living were also negatively correlated with prefrontal WMLs (r=-0.36, p<.05). WMLs volumes were significantly higher in the frontal region (p<.05) in PD. Stepwise regression showed that prefrontal WMLs alone significantly predicted UPDRS (b=15243, F(1,37)=9.635, p=.004) and TBS (b=-6689, F(1,29)=5.518, p=.026), while frontal WMLs alone significantly predicted MoCA (b=-905, F(1,32)=30.69, p<.001) and FAB scores (b=-361, F(1,32)=14.07, p= .001).
Discussion
The presence of WMLs in both PD and controls strongly correlated with motor and gait impairments, and deficits in attention and executive function. Frontal lobe functions are more severely affected by WMLs in PD, demonstrating the importance of connectivity within the frontal and prefrontal regions on motor and gait. On the other hand, higher-order brain functions (e.g., executive function) are more vulnerable to WMLs changes that affect the connectivity of parietal and temporal association cortices. We did not find a differential impact of periventricular WMLs on clinical assessments which discards the hypothesis that periventricular WMLs may characterize rapid neurodegeneration in severe PD16. Future studies will aim at: i) quantifying factor loadings on WMLs location; ii) evaluating the impact of periventricular WMLs on other PD motor subtypes and in other specific cognitive domains.Conclusion
WMLs volume and spatial location influences the effect of white matter damage on regional cortical connectivity with differing impact on motor and cognitive functions. Strong associations between WMLs, particularly within the frontal/prefrontal region, and both motor/gait and cognitive dysfunction may be due to a disruption in neuronal connectivity. A fully automated volumetric quantification of WMLs load, especially within the frontal/prefrontal region, can predict severity of PD symptoms.Acknowledgements
*Equal contributions
We thank the National Medical Research Council and Siemens for their support.
References
1. Murray ME, Senjem ML, Petersen RC, et al. Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Archives of Neurology. 2010;67(11):1379-1385.
2. Baezner H, Blahak C, Poggesi A, et al. Association of gait and balance disorders with age-related white matter changes: The LADIS Study. Neurology. March 18, 2008 2008;70(12):935-942.
3. Bohnen NI, Albin RL. White matter lesions in Parkinson disease. Nature Reviews Neurology. 02/22/online 2011;7:229.
4. Lee, S. J., Kim, J. S., Lee, K. S., An, J. Y., Kim, W., Kim, Y. I., ... & Jung, S. L. (2009). The severity of leukoaraiosis correlates with the clinical phenotype of Parkinson’s disease. Archives of gerontology and geriatrics, 49(2), 255-259.
5. G E Alexander, M R DeLong a, Strick PL. Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex. Annual Review of Neuroscience. 1986;9(1):357-381.
6. Gattellaro G, Minati L, Grisoli M, et al. White Matter Involvement in Idiopathic Parkinson Disease: A Diffusion Tensor Imaging Study. American Journal of Neuroradiology. 2009;30(6):1222-1226.
7. Bohnen NI, Bogan CW, Müller MLTM. Frontal and periventricular brain white matter lesions and cortical deafferentation of cholinergic and other neuromodulatory axonal projections. European neurological journal. 2009;1(1):33-50.
8. Dalaker TO, Larsen JP, Bergsland N, et al. Brain atrophy and white matter hyperintensities in early Parkinson's disease. Movement Disorders. 2009;24(15):2233-2241.
9. Lee, S. J., Kim, J. S., Yoo, J. Y., Song, I. U., Kim, B. S., Jung, S. L., ... & Lee, K. S. (2010). Influence of white matter hyperintensities on the cognition of patients with Parkinson disease. Alzheimer Disease & Associated Disorders, 24(3), 227-233.
10. Griffanti, L., Zamboni, G., Khan, A., Li, L., Bonifacio, G., Sundaresan, V., ... & Jenkinson, M. (2016). BIANCA (Brain Intensity AbNormality Classification Algorithm): A new tool for automated segmentation of white matter hyperintensities. Neuroimage, 141, 191-205.
11. Schmitter D, Roche A, Maréchal B, et al. An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer’s disease. NeuroImage : Clinical. 2015;7:7-17. doi:10.1016/j.nicl.2014.11.001.
12. Fartaria MJ, Bonnier G, Roche A, et al. Automated detection of white matter and cortical lesions in early stages of multiple sclerosis. Journal of Magnetic Resonance Imaging. 2016;43(6):1445-1454.
13. Anbeek P, Vincken KL, van Osch MJP, Bisschops RHC, van der Grond J. Probabilistic segmentation of white matter lesions in MR imaging. NeuroImage. 2004/03/01/ 2004;21(3):1037-1044.
14. Datta S, Narayana PA. A comprehensive approach to the segmentation of multichannel three-dimensional MR brain images in multiple sclerosis. NeuroImage: Clinical. 2013/01/01/ 2013;2(Supplement C):184-196.
15. Clogg CC, Petkova E, Haritou A. Statistical Methods for Comparing Regression Coefficients Between Models. American Journal of Sociology. 1995;100(5):1261-1293.
16. Piccini P, Pavese N, Canapicchi R, et al. White matter hyperintensities in parkinson's disease: Clinical correlations. Archives of Neurology. 1995;52(2):191-194.