3625
Overlap of R2* map based SN and SNpc defined by neuromelanin-sensitive MRI in Parkinson’s Disease: A promising diagnostic biomarker1Department of Radiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 2Center for Advanced Neuroimaging, University of California, Riverside, Riverside, CA, United States, 3Department of Neurology and Institute of Neurology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 4Department of Electrical Engineering and Computer Sciences, & Helen Wills Neuroscience Institute, University of California, Berkeley, San Francisco, CA, United States, 5MR Research, GE Healthcare, Shanghai, China, Shanghai, China, 6Department of Bioengineering University of California, Riverside, Riverside, CA, United States
Synopsis
There is an urgent need for developing diagnostic imaging biomarkers for Parkinson’s Disease (PD). In this work, we applied a standardized substantia nigra pars compacta (SNpc) mask based on neuromelanin-sensitive MR images from healthy subjects to investigate the diagnostic performance of the SNpc overlap percentage and R2* in the SNpc overlap in PD. R2* in the SNpc overlap volume was increased in PD patients as compared to controls. Furthermore, it was significantly positively correlated with the disease duration in PD. We found an excellent diagnostic accuracy for the SNpc overlap percentage (AUC, 0.927) in PD.
Introduction
Parkinson’s disease (PD) affects about seven million people worldwide. Although the exact cause of the disease remains unclear, one possible cause is increased iron accumulation in the substantia nigra (SN) which leads to neurotoxic consequences such as oxidative damage and cell death1. SN pars compacta (SNpc) is composed of closely packed dopaminergic neurons. Histopathological studies have shown that iron accumulation is severe and mainly occurs in the neuromelanin granules within SNpc in PD2, 3. Further, approximately 50% of melanized dopamine neurons in SNpc have been lost by the time of PD diagnosis4. However, the current diagnostic criteria for PD mainly rely on the presence of motor symptoms and a set of clinical assessments 5, 6. Therefore, there is an urgent need for prodromal imaging biomarkers.
Difficulty in identifying SN or SNpc on conventional MR images has been a confounding source of variability in previous studies7-9. Even with the improved resolution available at 3T, defining the border between the SNpc and the SNr on T2-weighted images has been difficult and remains controversial10. Previous studies have shown that neuromelanin-sensitive MRI provides contrast sensitive of neuromelanin granules in SNpc11. Here, we apply, a standardized SNpc mask, based on neuromelanin-sensitive MR images from healthy subjects12, to elucidate region-specific iron deposition in SNpc after PD onset.
Methods
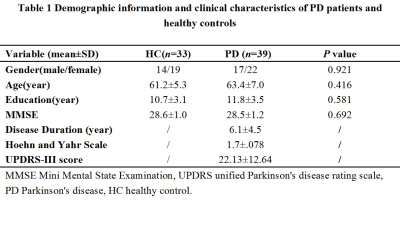
Thirty-nine PD patients were recruited from the movement disorder outpatient clinic diagnosed according to the UK Brain Bank criteria13. 33 healthy controls were recruited. The current study was approved by the local ethics committee.
MRI scanning
All subjects underwent scanning with at 3T (Signa HDxt,GE Healthcare) equipped with an eight-channel head coil. A 3D multi-echo GRE sequence was utilized to obtain T2*-weighted images. Imaging parameters were: TR=59.3ms; echoes=16; TE1=2.7ms; TE spacing=2.9ms; FA=12°; FOV=22×22cm2; resolution=0.86×0.86×1.0mm3; acceleration factor=2; and total acquisition time=10minutes 42seconds. Whole brain anatomical images were acquired with a T1-weighted fast spoiled gradient echo sequence for common space registration. Imaging parameters for this sequence were: TR=5.5ms; TE=1.7ms; IT=450ms, resolution = 1×1×1 mm3; FA =12.
Image processing
R2* values were calculated using a mono-exponential fit to the signal decay. The overlap possibility between the whole SNpc (SNpc volume defined by neuromelanin sensitive MR) and R2* hyperintense SN (using a threshold of the mean plus three times the standard deviations) is defined as below, and offers a means to evaluate iron deposition in SNpc. SNpc overlap percentage =Volume (SNNM intersection with SNhyperintense)/Volume(SNNM) where SNNM and SNhyperintense denote the NM MRI based SNpc and R2* hyperintense SN volumes, respectively.
Statistical analysis
A chi-square test or an independent two-sample t-test was performed to compare demographic and clinical features between groups. R2* in the SNpc overlap volume and the SNpc overlap percentage between groups were compared using a two-sample t-test. Correlation analysis was performed between R2* in the SNpc overlap volume and the SNpc overlap percentage with disease duration and UPDRS-III score. Diagnostic performance of R2* values in the SNpc overlap volume and the SNpc overlap percentage was performed using a receiver operating characteristics (ROC) analysis. The threshold of statistical significance was set to p<0.05.
Results
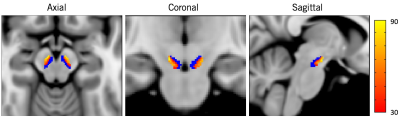
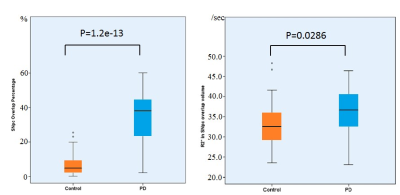
All the demographic and clinical data for PD and control groups are shown in Table1. The SNpc overlap volume corresponds to similar regions in SNpc showing reduced NM-sensitive contrast, and presumably NM depletion14 (Figure1) specifically in the lateral ventral tier, which is corroborated by histology4. Significant R2* increases were seen in PD in the SNpc overlap volume (PD: 35.9±5.9, controls: 33.2±5.7, p=0.0286). The SNpc overlap percentage was significantly increased in PD patients as compared to controls (PD: 0.33±0.15;controls: 0.07±0.07, p=1.2e-13) (Figure2).
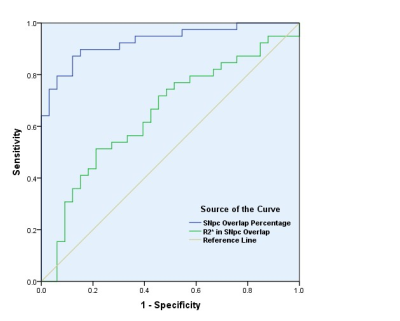
The SNpc overlap volume R2* (r=0.420; p=0.008) was positively correlated with disease duration in PD patients. ROC analysis of R2* in the SNpc overlap volume and the SNpc overlap percentage in PD and controls found an area under the curve (AUC) of 0.661 (95% CI: 0.531-0.791; P=0.021), and 0.927 (95% CI: 0.867-0.988; P=0.000), respectively (Figure3).
Discussion and conclusion
In this work, we used this standardized SNpc ROI to avoid the problem of variability in SN ROIs used in previous studies14, 15. We found an excellent diagnostic accuracy for the SNpc overlap percentage in PD. This SNpc overlap percentage applied here is based on the overlap between neuromelanin-sensitive and iron-sensitive MRI contrasts, and may represent a promising tool to investigate the nigral PD biology in vivo.
R2* values in the SNpc overlap volume is significantly increased in PD patients, indicating increased iron deposition. We found significant correlation of R2* values in the SNpc overlap volume with disease severity in PD patients which suggests that an increase in SNpc iron content may reflect a marker for disease progression.
Acknowledgements
XH and JL receive support from the Michael J Fox Foundation (MJF 10854). NH and FY receive support from Shanghai science grant (17411952700). Thank you to Dr. Ewart Mark Haacke for his critical review of the abstract.References
1. Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. The Lancet Neurology 2014;13:1045-1060. 2. Sian-Hulsmann J, Mandel S, Youdim MB, Riederer P. The relevance of iron in the pathogenesis of Parkinson's disease. Journal of neurochemistry 2011;118:939-957. 3. Wypijewska A, Galazka-Friedman J, Bauminger ER, et al. Iron and reactive oxygen species activity in parkinsonian substantia nigra. Parkinsonism Relat Disord 2010;16:329-333. 4. Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 1991;114 ( Pt 5):2283-2301. 5. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. Journal of neurology, neurosurgery, and psychiatry 1992;55:181-184. 6. Caslake R, Moore JN, Gordon JC, Harris CE, Counsell C. Changes in diagnosis with follow-up in an incident cohort of patients with parkinsonism. Journal of neurology, neurosurgery, and psychiatry 2008;79:1202-1207. 7. Vaillancourt DE, Spraker MB, Prodoehl J, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology 2009;72:1378-1384. 8. Martin WR, Wieler M, Gee M. Midbrain iron content in early Parkinson disease: a potential biomarker of disease status. Neurology 2008;70:1411-1417. 9. Minati L, Grisoli M, Carella F, De Simone T, Bruzzone MG, Savoiardo M. Imaging degeneration of the substantia nigra in Parkinson disease with inversion-recovery MR imaging. AJNR American journal of neuroradiology 2007;28:309-313. 10. Oikawa H, Sasaki M, Tamakawa Y, Ehara S, Tohyama K. The substantia nigra in Parkinson disease: proton density-weighted spin-echo and fast short inversion time inversion-recovery MR findings. AJNR American journal of neuroradiology 2002;23:1747-1756. 11. Langley J, Huddleston DE, Chen X, Sedlacik J, Zachariah N, Hu X. A multicontrast approach for comprehensive imaging of substantia nigra. Neuroimage 2015;112:7-13. 12. Langley J, Huddleston DE, Sedlacik J, Boelmans K, Hu XP. Parkinson's disease-related increase of T2*-weighted hypointensity in substantia nigra pars compacta. Movement disorders : official journal of the Movement Disorder Society 2017;32:441-449. 13. Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet 2009;373:2055-2066. 14. Huddleston DE, Langley J, Sedlacik J, Boelmans K, Factor SA, Hu XP. In vivo detection of lateral-ventral tier nigral degeneration in Parkinson's disease. Hum Brain Mapp 2017;38:2627-2634. 15. Langley J, Huddleston DE, Merritt M, et al. Diffusion tensor imaging of the substantia nigra in Parkinson's disease revisited. Hum Brain Mapp 2016;37:2547-2556.Figures