3623
CORRELATION OF IMMUNE ACTIVATION BIOMARKERS AND MRS MEASURES IN ACUTE HIV INFECTION1Tropical Medicine, University of Hawaii, kapolei, HI, United States, 2Tropical Medicine, University of Hawaii, Honolulu, HI, United States, 3Memory and Aging Center, Department of Neurology, University of California, San Francisco, CA, United States, 4SEARCH, The Thai Red Cross AIDS Research Centre, Bangkok, Thailand, 5U.S. Military HIV Research Program, Silver Spring, MD, United States, 6Office of Biostatistics & Quantitative Health Sciences, University of Hawaii, Honolulu, HI, United States, 7Missouri Institute of Mental Health, St. Louis, MO, United States, 8Neurology, Yale University, New Haven, CT, United States, 9Memory and Aging Center, San Francisco, CA, United States, 10Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, United States
Synopsis
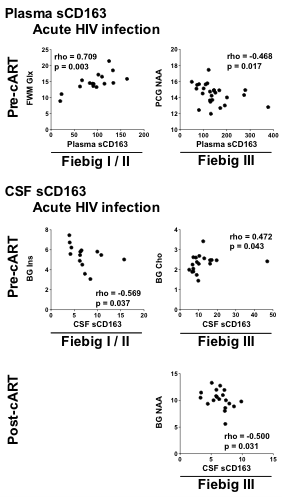
Proton MRS was performed in 51 acute HIV participants. We observed Inverse correlations between sCD163 and markers of neuronal integrity (BG and PCG NAA) in select brain regions suggests regional variability to neuronal injury in AHI. Further, correlations between CSF sCD163 and BG Cho and mI reveal that membrane turnover activity and
INTRODUCTION
Soluble CD163 (sCD163) shed by myeloid cells (monocytes/macrophages) is thought to be a marker of HIV-associated immune activation [1].Elevated levels of plasma sCD163 in plasma are associated with poor neuropsychological testing performance in chronic and primary HIV infection [2-4]. Here, we examined brain changes associated with sCD163 levels longitudinally in participants who initiate treatment during early acute infection (AHI).METHODS
We completed proton magnetic resonance spectroscopy (MRS) using a 1.5T scanner in Thai HIV-infected participants who started combination antiretroviral therapy (cART) during AHI with Fiebig stages of I-III. The MRS examinations were performed before cART at a mean of 20 days of estimated infection. Chronic HIV (CHI) and HIV-uninfected (HIV-) participants served as controls. MRS voxels (TE=35ms and TR=1.5s) were placed in the basal ganglia (BG), frontal grey matter (FGM), frontal white matter (FWM), and posterior cingulate gyrus (PCG) to measure myoinositol (mI), choline (tCho), creatine (Cr), N-acetyl aspartate (NAA), and glutamate/glutamine (Glx). An LCModel was used to quantify brain metabolites expressed in institution unit (IU). sCD163 was quantified in plasma and CSF by singleplex ELISA (Biorad, Hercules, USA). Participants completed a brief battery of neuropsychological tests examining fine motor dexterity, psychomotor speed, and executive function. Raw scores were converted to standardized scores and the individual scores were aggregated to create a global measure of neuropsychological testing performance. Exploratory statistical analyses were performed using R version 3.2.2 or Prism GraphPad version 7 (GraphPad Software, San Diago, CA, USA).RESULTS
The 51 AHI participants (>90% male; median age 31 years) exhibited median sCD163 levels pre- and post-cART in Fiebig I/II that were comparable to HIV- participants (plasma: 97.9ng/ml vs 99.5ng/ml; CSF: 6.7ng/ml vs. 7.1ng/ml). By contrast, among AHI in Fiebig III, sCD163 levels were elevated pre-cART compared to HIV- individuals (plasma:135ng/ml; CSF:10ng/ml; p’s<0.01), prior to normalization with cART (plasma:90.1ng/ml; CSF:6.5ng/ml). MRS correlational analyses are summarized in Figure 1. In AHI pre-cART, we observed an inverse correlation between plasma sCD163 (p = 0.017) and PCG NAA and a direct correlation with FWM Glx (p = 0.003). We observed an inverse correlation between CSF sCD163 and BG mI (p = 0.037) and a direct correlation with BG Cho (p = 0.043). Post-cART an inverse correlation was observed between CSF sCD163 and BG NAA (p= 0.031).CONCLUSION
Inverse correlations between sCD163 and markers of neuronal integrity (BG and PCG NAA) in select brain regions suggests regional variability to neuronal injury in AHI. Further, correlations between CSF sCD163 and BG Cho and mI reveal that membrane turnover activity and glia activation that occur early in infection remain susceptible to CNS neuroinflammation. Our observation suggests select brain injury linked to myeloid activation persists even early ART in AHI arguing for additional interventions to halt detrimental neuroinflammation.Acknowledgements
The authors thank the SEARCH 010/RV 254, SEARCH011 and SEARCH013 protocol teams.References
1. Burdo, T.H., et al., Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis, 2011. 204(1): p. 154-63.
2. Burdo, T.H., et al., Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS, 2013. 27(9): p. 1387-95.
3. Imp, B.M., et al., Monocyte Activation Is Associated With Worse Cognitive Performance in HIV-Infected Women With Virologic Suppression. J Infect Dis, 2017. 215(1): p. 114-121.
4. Burdo, T.H., et al., Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis, 2011. 204(8): p. 1227-36.