3621
Distinct mechanism underlying patients who might benefit from edaravone or those may not - a combined quantitative susceptibility mapping and DTI study1Department of Medical Imaging, the First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China, 22GE Healthcare, MR Research China, Beijing, China, 3School of Life Science and Technology, Xi'an Jiaontong University, the Key Laboratory of Biomedical Information Engeering, Ministry of Education, Xi‘an’, China
Synopsis
The coexistence of multiple complicated pathological mechanisms, and lack of valid biomarker for treatment effects, remains to be main challenges that retarded ALS clinical trials. The approve of edaravone in treating ALS confirmed the importance of oxidative stress in ALS pathology. However, only a subgroup of patients would benefit with edaravone. We used QSM and DTI to explore the different mechanism that underlying those two cohorts. Our study revealed that neuroinflammation-prominent pathology and age-dependent oxidative stress might a feature for patients benefit with
Purpose
The coexistence of multiple complicated pathological mechanisms, as well as lack of valid biomarker for treatment effects, remains to be main challenges that retard ALS clinical trials1. The approve of edaravone, a scavenger of reactive oxygen species, in treating ALS confirmed the importance of oxidative stress in ALS pathology2. However, only a subgroup of patients would benefit with edaravone therapy. The evaluation of the potential different mechanism in patients who would benefit or not benefit with edaravone would not only deepen our understanding of treatment responds, but also help to make critical strategies for future clinical trials. Iron-related oxidative stress as pontential mechanism has been observed in ALS studies, both in human and animal studies3. Quantitative susceptibility mapping has been shown to be a reliable method that could reflect brain iron level, with superior anatomical contrast. Therefore, in the current study, we aimed to use QSM to evaluate the potential difference between patients who fulfill or not fulfill the inclusion criteria of edaravone. Additionally, we used the multiple measurements of DTI that related with specific pathological changes to explore the underlying difference.Methods
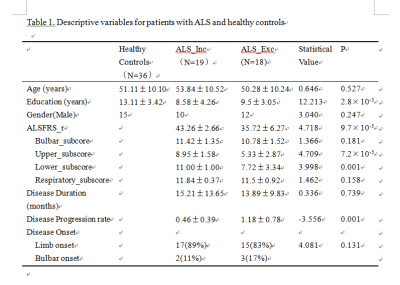
We enrolled 37 ALS patients and 36 age- and gender-matched healthy controls. ALS patients were subsequently divided into two subgroups according to the inclusion criteria of edaravone. Table 1 illustrated the demorgraphic and clinical measurements in each groups. All subjects underwent gradient-echo sequence, DTI and 3D T1WI scan (GE 3.0T, HDxt). The anatomical regions in the frontal lobe and four subcortical structures, which frequently involved in ALS pathology, were served as regions of interests. Detailed methods and procedures listed in Figure 1. For QSM analysis, multivariates analysis of covariates adjusted for age and gender were used, with Bonferroni test used for post hoc comparisons. Standardized TBSS was used to assess white matter changes among groups, controlling for age and gender. Pearson’s correlation was used to evaluate relationship between clinical measurements and neuroimaging findings.Results
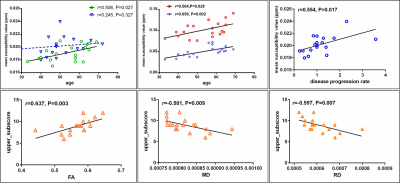
Patients in ALS_Inc subgroup showed increased iron concentration mainly in putamen and globus pallidum, both of which positively related with age (Figure 2,4b). Increased iron level has been found in the primary motor cortex (PMC), frontal opercular and inferior frontal gyrus in patients at ALS_Exc subgroup (Figure 2). Although patients at ALS_Inc subgroup did not show excessive iron concentration in PMC as compared with normal controls, statistical significant reduced iron level was found in these patients, compared with ALS_Exc subgroup. Additionally, for patients at ALS_Inc subgroup, iron concentration in PMC was positively correlated with age, while no relationship between age and PMC iron level in patients at ALS_Exc subgroup was detected (Figure 4a). TBSS analysis showed extensive white matter degeneration that characterized with reduced FA, increased MD, RD and AD, in patients at ALS_Inc subgroup, which indicative of neuroinflammation. The involved regions mainly consisted of corticospinal tract, corpus collasom, and their frontal coronal radiate. For patients at ALS_Exc subgroup, circumscribed FA reduction was found in corticospinal tract and body of corpus callosum. Correlation analysis revealed positive relationship between iron concentration in PMC and disease progression rate in patients at ALS_Exc subgroup. For patients at ALS_Inc subgroup, only FA, MD and RD values that showed group differences were correlated with upper limb subscore of ALSFRS-r.Discussion
Increased iron-mediated oxidative stress and neuroinflammation are important pathological mechanism that participate motor neuron degeneration. For patients at ALS_Inc, iron accumulation in the globus pallidum and putamen, and their medium correlation with age were more similar to those found in aging process. In normal aging, higher iron amount could be found in the putamen and pallidum4. Additionally, obvious neuroinflammation changes in extensive white matter in patients at ALS_Inc subgroup, rather than iron-related oxidative stress in the PMC that related with motor function score, highlighted the close causal relationship between increased inflammation and oxidative stress5. Thus, we concluded that for patients who might benefit with edaravone therapy, the administration of edaravone may alleviate secondary oxidative stress caused by increased inflammation. While for patients at ALS_Exc subgroup, the close relationship between iron concentration in PMC and faster disease progression rate indicated that iron-mediated pathology accelerated disease progression, independent with detectable white matter alterations. The non-benefit inference from edaravone therapy for these patients might cause by inadequate drug efficacy, or ferroptosis, which characterized with iron-dependent lipid peroxidation that results in cell death6.Conclusion
Distinct pathology-dominant features in ALS patients with different clinical manifestation might clarify the potential different treatment responds。The use of specific pathology sensitive neuroimaging biomarker for treatment effects is desirable for individualized therapy strategies in future.Acknowledgements
noneReferences
1. Mitsumoto H, Brooks BR, Silani V. Clinical trials in amyotrophic lateral sclerosis: why so many negative trials and how can trials be improved?[J]. Lancet Neurol, 2014, 13 (11): 1127-1138. 2. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial[J]. Lancet Neurol, 2017, 16 (7): 505-512. 3. Ferraiuolo L, Kirby J, Grierson AJ, et al. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis[J]. Nat Rev Neurol, 2011, 7 (11): 616-630. 4. Acosta-Cabronero J, Betts MJ, Cardenas-Blanco A, et al. In Vivo MRI Mapping of Brain Iron Deposition across the Adult Lifespan[J]. J Neurosci, 2016, 36 (2): 364-374. 5. Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease[J]. Lancet Neurol, 2011, 10 (3): 253-263. 6. Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease[J]. Cell, 2017, 171 (2): 273-285.Figures
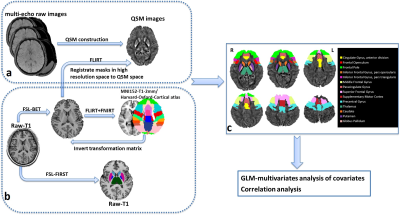
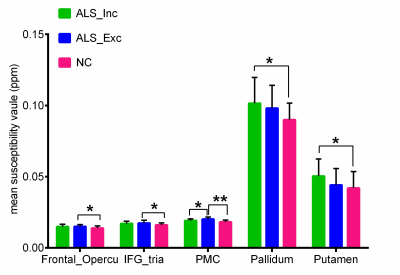
Figure 2. group comparison of QSM values. * P < 0.05; ** P<0.001.