3619
Quantitative Magnetic Resonance Imaging in diabetes: inflammation, oedema and neurodegeneration1Institute of Neuroscience and Medicine 4, Medical Imaging Physics, Research Centre Juelich, Juelich, Germany, 2Institute of Neuroscience and Medicine 1, Research Centre Juelich, Juelich, Germany
Synopsis
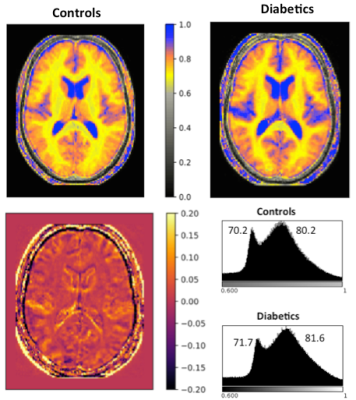
Type II diabetes is one of the most important metabolic disorders for public health with around 8% prevalence in European population (11% in the US). We report here for the first time a generalized increase in brain water content of ~ 2% in type II diabetics compared to age- and gender-matched controls, supporting the presence of neuroinflammation in diabetes. Several other quantitative measures are investigated (T1, T2*, MT parameters, magnetic susceptibility and diffusion kurtosis) as well as region-based volume, area and cortical thickness. Regions with significant changes in a large number of quantitative parameters are identified.
Introduction
Type II diabetes mellitus (T2DM) is one of the most important metabolic disorders for public health [1], reaching ~8% prevalence in normal european population (11% in the US). The central nervous system is affected by T2DM; among others, myelopathy and encephalopathy, [2] cognitive impairment and increased risk of dementia [3] have been described. Exacerbation of neurodegeneration by hyperglycemia is reported in T2DM [4]. Total grey, white matter and hippocampal are atrofied in diabetic patients [5]. Furthermore, diabetes has been associated with low grade systemic inflammation and neuroinflammatory processes [6], but quantitative in-vivo measurements of brain inflammation were still lacking. We report here for the first time in-vivo non-invasive detection of brain oedema levels in type II diabetic patients. Other quantitative parameters reflecting myelination, iron content and presence of cellular compartments and membranes are also investigated.Materials and Methods
The preliminary results include 7 subjects with T2DM and 7 age, gender and education-matched controls not affected by metabolic syndrome (all male, age=69.6±5.5 and 67.3±5.8). The subjects have been drawn from the population-based cohort study 1000BRAINS, which aims at assessing the influence of environmental and genetic factors on the variability of structure and function of the aging brain[7]. Only subjects without notable white matter hyperintensities were selected. The quantitative parameters H2O, T1, T2* and MT measures (magnetisation transfer ratio MTR, exchange rate kex , bound proton fraction fbound) were derived using a 3D 2-point method similar to that described in [8], to which a magnetisation transfer was added. The protocol consists of five sequences: an M0-weighted (α=7◦) and a T1-weighted multi-echo gradient echo (meGRE) (α=40◦) both with and without MT (off-resonance frequency -1.5kHz), respectively, and an actual flip angle sequence [9] (AFI) (α=40◦) to map the transmit field, B1+. Other imaging parameter for the meGREs (AFI) were set as follows: TR=50ms (150ms), 18 echoes (12 for MT preparation), 1x1x2mm3 resolution (2.8x2.8x4.0mm3), matrix size 162x192x96 (54x64x48), bandwidth 650Hz/px (330Hz/px), phase and slice partial Fourier 6/8, parallel imaging using GRAPPA factor 2 with 24 reference lines. The total acquisition time for the quantitative protocol was TA=14:20min. Magnitude and phase images were saved and processed with in-house software (written in python) and STISuite [10]. In addition, an extensive diffusion protocol (b-values of 1000 and 2700 s/mm2, 60+120 directions) and a standard T1-weighted anatomical scan (MP-RAGE) were acquired as described in [7]. VBM analysis using CAT12/SPM12 was performed (2 sample t-test, cluster size >100 voxels, p<0.01 uncorrected) using the anatomical scans. Kurtosis tensor analysis was performed using MIBCA [11]. The quantitative maps were registered to the MNI template and averaged for each group (T2DM vs controls) and the two averaged sets of parametric maps were compared. Parcellation of the brain in each individual space using FreeSurfer defined ROIs for region-based comparison. Changes in structural connectivity of the brain was performed with MIBCA using tractography based on diffusion kurtosis tensor analysis [9]. Correlations between pairs of parameters were investigated.Results and Discussion
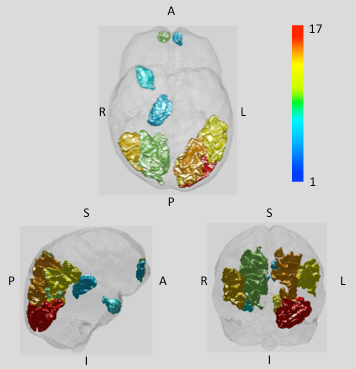
We focus the discussion on findings related to water content, VBM and regions showing highest degree of changes of quantitative parameters. Fig. 1 shows water content maps of a selected slice from the group-averaged volumes (left, T2DM; right, controls) and the corresponding histograms. The diabetic brain displays a shift in the water content distribution; its mean value over the whole brain is 2%. The VBM analysis revealed hypertrofic regions in diabetes to be: anterior and posterior lobe of right cerebellum, anterior lobe of left cerebellum, right precentral gyrus and the superior temporal gyrus/insula. Atrophy was found in the inferior, medial, superior frontal gyri (bilateral), orbitofrontal cortex, right parahippocampal gyrus, anterior and middle cingulum, bilateral fusiform gyri and lingual gyrus. Interestingly, changes in the different quantitative parameters based on the 3D protocol did not reach the significance level (p<0.05) in any of the regions highlighted by VBM, but the trend showed a reduced increase in water content compared to the noticed global increase. Global white and grey matter, the thalamus and temporal white matter showed significant changes, followed closely by changes in corpus callosum (CC) and hypothalamus (HT) with 0.05<p<0.1. Decreased susceptibility values (more paramagnetic) were found in the basal ganglia (pallidum, putamen, caudate), as usually associated with neurodegenerative diseases. We summarise our qMRI findings in Fig. 2, where regions defined by Freesurfer are colour coded by the number of parameters with significant changes (p<0.1)Conclusions
We report here for the first time a generalized increase in brain water content in type II diabetics compared to age- and gender-matched controls. A substantial number of regions displaying changes in several quantitative parameters are identified.Acknowledgements
This work is funded in part by the Helmholtz Alliance ICEMED - Imaging and Curing Environmental Metabolic Diseases, through the Initiative and Network Fund of the Helmholtz Association.References
1. L. Guariguata, D.R. Whiting, I. Hambleton, J. Beagley, U. Linnenkamp, J.E. Shaw, Global estimates of diabetes prevalence for 2013 and projections for 2035, In Diabetes Research and Clinical Practice, Volume 103, Issue 2, 2014, Pages 137-149, ISSN 0168-8227, https://doi.org/10.1016/j.diabres.2013.11.002.
2. DeJong RN. CNS manifestations of diabetes mellitus. Postgrad Med. 1977 Jan;61(1):101-7.
3. Gispen WH, Biessels GJ. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000;23:542–549.
4. Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R (2001) Diabetes mellitus and risk of Alzheimer's disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol 154: 635-641.
5. Moran, C., Phan, T. G., Chen, J., Blizzard, L., Beare, R., Venn, A., … Srikanth, V. (2013). Brain atrophy in type 2 diabetes: Regional distribution and influence on cognition. Diabetes Care, 36(12), 4036–4042.
6. Shukla, V., Shakya, A. K., Perez-Pinzon, M. A., & Dave, K. R. (2017). Cerebral ischemic damage in diabetes: an inflammatory perspective. Journal of Neuroinflammation, 14(1), 21.
7. Caspers, S., Moebus, S., Lux, S., Pundt, N., Schütz, H., Mühleisen, T. W., … Amunts, K. (2014). Studying variability in human brain aging in a population-based German cohort-rationale and design of 1000BRAINS. Frontiers in Aging Neuroscience, 6(JUL), 1–14.
8. Schall, M., Zimmermann, M., Iordanishvili, E., Gu, Y., Shah, N.J., Oros-Peusquens, A.-M., Quantitative In Vivo Imaging Using a 3D Two-Point Method, Magn Reson Mater Phy (2017), abstract ID 247
9. Yarnykh, V., Actual flip-angle imaging in the pulsed steady state: A method for rapid three-dimensional mapping of the transmitted radiofrequency field, Magnetic Resonance in Medicine, 2007, 57(1):192-200
10. https://people.eecs.berkeley.edu/~chunlei.liu/software.html 11. Neto Henriques et al, MAGMA, 2015
Figures