3616
Regional cortical folding morphometry in Friedreich ataxia using Laplace Beltrami based gyrification index1School of Psychological Sciences and Monash Institute of Cognitive and Clinical Neurosciences, Monash University, Melbourne, Australia, 2Monash Biomedical Imaging, Monash University, Melbourne, Australia, 3Department of Neurology, RWTH Aachen University, Aachen, Germany, 4JARA - Translational Brain Medicine, Aachen and Juelich, Germany, 5Bruce Lefroy Centre for Genetic Health Research, Murdoch Childrens Research Institute, Melbourne, Australia, 6Clinical Genetics, Austin Health, Melbourne, Australia, 7Department of Paediatrics, The University of Melbourne, Melbourne, Australia, 8Department of Medicine, Monash University, Melbourne, Australia
Synopsis
Friedreich ataxia (FRDA) is an inherited neurodegenerative disorder mainly affecting the spinal cord and dentate nuclei of the cerebellum. Although there is growing evidence of cerebral atrophy and cortical thinning in FRDA, no research has investigated the pattern of cortical folding (gyrification) in the disorder. We have proposed a new MRI analysis technique, Laplace Beltrami based gyrification index (LB-GI), and validated its use in individuals with FRDA. Preliminary results reveal significantly increased regional gyrification in the motor cortex in individuals with FRDA, compared to healthy controls. Overall, our results demonstrate that LB-GI is a sensitive technique which requires further investigation as a potential neuroimaging marker of disease progression in FRDA.
Introduction:
Friedreich ataxia (FRDA) is an autosomal recessive neurodegenerative disorder, mainly affecting the spinal cord and dentate nuclei of the cerebellum1. The extent and pattern of cerebral cortex involvement, however, is less clear with findings of both structural volume loss and cortical thinning1. The gyrification index (GI), a measure of cortical folding morphometry, has been widely used as a sensitive marker to detect abnormal cortical patterns in a number of neurodegenerative disorders2,3, but has not been previously studied in FRDA.
We have developed a novel cortical GI based on Laplace Beltrami (LB) eigenfunctions, which we entitled Laplace Beltrami based gyrification index (LB-GI)4. As we have demonstrated4, LB-GI embodies both cortical curvature and sulcal depth. The applicability of this measure was examined on developing fetal brains. We have since built upon the previous method and propose using LB-GI for highly convoluted adult brains. Our aim was to investigate LB-GI sensitivity to detect abnormalities of cortical folding morphometry in neurodegenerative disease using FRDA as an exemplar.
Methods: Participants and MRI data acquisition
Twenty nine individuals with genetically confirmed FRDA (mean age: 41±13 years, 17 males, mean GAA repeats on the shorter allele: 543±232, longer allele: 861±241; disease duration: 18.7±9.6 years) were recruited as part of the IMAGE-FRDA study at Monash University (Melbourne). The control group comprised 30 healthy volunteers matched for age and gender (mean age: 39 ± 13 years, 16 males), with no history of neurological or psychiatric diseases.
All individuals were scanned on a 3 Tesla Siemens Skyra scanner with a 32-channel head coil. High-resolution T1-weighted images were acquired, over 3.5 min, using magnetization prepared rapid gradient-echo (MP-RAGE) imaging with TE/TR = 2.19 /1900 ms, flip = 9°, matrix size = 256X256, 176 sagittal slices, and 1.0 mm isotropic voxels.
Methods: Regional analysis of gyrification
The cortical surface
reconstruction and parcellation were conducted in FreeSurfer5. The localised
LB-GI values were then calculated on the generated pial cortical surface using
the proposed LB-GI pipeline, included the following steps: 1) compute the level-sets
of the first, second and third non-constant LB eigenfunction; 2) estimate mean
curvature of the cortical surface and projecting the values on each level-set;
3) automatically detect gyral points on each level sets; and 4) compute LB-GI
on each point on level sets as the relative curvature with reference to the
neighbouring gyral points and then
map the values to the triangulated surface.
The regional LB-GI were defined as the mean LB-GI across all vertices within the gyral based Desikan-Killiany parcellations6. Between-group differences were assessed using analyses of covariance (ANCOVA), adjusted for age and gender, for each of the 34 cortical regions of interest (ROIs) in each hemisphere. False discovery rate (FDR) correction at p<0.05 was conducted to adjust for multiple comparisons. Finally, where there were regional LB-GI differences between groups, Pearson correlation coefficients were used to assess any associations between these regions and GAA repeat length on both alleles and disease duration in individuals with FRDA.
Results:
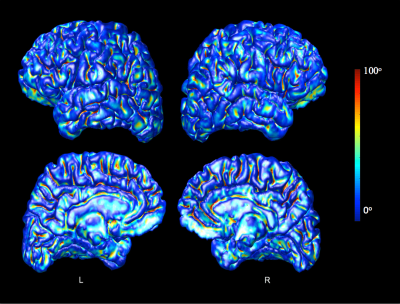
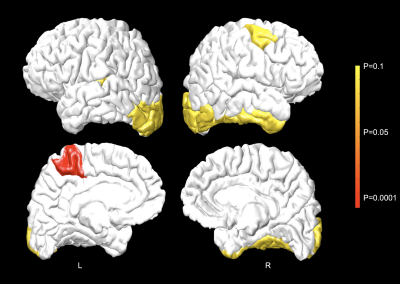
The LB-GI values computed on pial surface of a randomly selected healthy control are presented in Fig. 1. Maximal LB-GI values are assigned to highly curved and deep sulcal regions. Fig. 2 shows pial cortical regions with significance LB-GI differences between groups. The left para-central lobule, on the site of primary motor cortex, shows significantly (p<0.0001) higher regional LB-GI in individuals with FRDA compared to controls. A trend of regional gyrification increase is further observed in left lateral occipital and right fusiform, inferior temporal, lateral occipital and caudal middle frontal areas (0.05<p<0.1). This, however, did not withstand FDR correction. No significant correlations were found between cortical areas showing LB-GI increases with GAA repeat lengths on both alleles or disease duration in FRDA.Discussion and Conclusion:
In this study, we proposed a sensitive measure of gyrification, LB-GI, for the study of highly convoluted adult brains. this measure was introduced to detect cortical folding abnormalities in individuals with FRDA, but it could be applicable to other neurodegenerative disorders. We showed for the first time elevated LB-GI in individuals with FRDA as compared to control participants, indicating altered cortical folding morphometry, deeper sulci and higher cortical curvature, possibly related to cortical atrophy in FRDA. Overall, our results accord with previous studies showing cortical thinning in frontal, temporal and occipital lobes in FRDA7,8. These findings provide further evidence for cerebral cortex involvement in FRDA. Future longitudinal research is required to examine LB-GI associations with clinical measures such as disease progression.Acknowledgements
No acknowledgement found.References
1. Selvadurai L, Harding I, Corben L, et al. Cerebral abnormalities in Friedreich ataxia: A review. Neurosci. Biobehav. Rev. 2017; 1-13.
2. Nordahl C. W, Dierker D, Mostafavi I, et al. Cortical folding abnormalities in autism revealed by surface-based morphometry. The J. Neurosci. 2007; 27(43):11725– 11735.
3. Schaer M, Schmitt J. E, Glaser B, et al. Abnormal patterns of cortical gyrification in velo-cardio-facial syndrome (deletion 22q11. 2): an MRI study. Psychiatry Res. Neuroimaging 2006; 146(1): 1–11.
4. Shishegar R, Manton J. H, Walker D. W, et al. Quantifying gyrification using Laplace Beltrami eigenfunction level-sets. ISBI 2015; 1272-1275.
5. Dale A. M, Fischl B, Sereno M. I. Cortical surface-based analysis: I. segmentation and surface reconstruction. NeuroImage 1999; 9(2):179–194.
6. Desikan R. S, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006; 31(3):968–980.
7. Selvadurai L, Harding I, Corben L, et al. Cerebral and cerebellar grey matter atrophy in Friedreich ataxia: the IMAGE-FRDA study. J. Neurol. 2016; 263(11):2215-2223
8. Rezende T, Silva C, Yassuda C, et al. Longitudinal magnetic resonance imaging study shows progressive pyramidal and callosal damage in Friedreich's ataxia. Mov. Disord. 2016; 31:70-78.
Figures