3612
Dose-dependent effects of citicoline on the visuomotor response and white matter integrity in the visual pathway after chronic intraocular pressure elevationYolandi van der Merwe1,2, William J Kohler3, Michael Krawchuk3, Xiaoling Yang2, Leon C Ho4, Yu Yu5, Ying Chau6, Christopher K Leung7, and Kevin C Chan2,8,9
1Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, United States, 2Department of Ophthalmology, University of Pittsburgh, Pittsburgh, PA, United States, 3Neuroimaging Laboratory, University of Pittsburgh, Pittsburgh, PA, United States, 4Department of Electrical Engineering, University of Hong Kong, Hong Kong, Hong Kong, 5Division of Biomedical Engineering, Hong Kong University of Science and Technology, Hong Kong, Hong Kong, 6Department of Chemical and Biological Engineering, Hong Kong University of Science and Technology, Hong Kong, Hong Kong, 7Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong, Hong Kong, Hong Kong, 8Department of Radiology, New York University, New York, NY, United States, 9Department of Ophthalmology, New York University, New York, NY, United States
Synopsis
Glaucoma is a neurodegenerative disease that can cause irreversible vision loss. Elevated intraocular pressure is a major risk factor for the glaucoma; however, the disease may still progress in some patients after lowering IOP. Citicoline has been suggested as a potential therapeutic to ameliorate damage caused by neurodegenerative diseases, including glaucoma, but its neuroprotective effects remain incompletely studied. In this study, we analyzed the dose-dependent effect of oral citicoline on visual behavior response and white matter integrity in a rodent model of glaucoma. The results show citicoline preserves visual behavior response and visual system integrity in a dose dependent manner.
Introduction
Glaucoma is a neurodegenerative disease that causes irreversible vison over time, making it the second leading cause of blindness worldwide. Elevated intraocular pressure (IOP) is a risk factor associated with glaucoma, and while lowering IOP is a clinically accepted method to slow the progression of the disease, degeneration of the visual system may still occur after lowering the IOP1. Citicoline is an endogenous compound that acts in the biosynthetic pathway of cellular membrane synthesis, and recent studies suggest citicoline may be improve visual function outcome in glaucoma patients2,3. In this study, we used an experimental glaucoma model to elevate IOP for up to 5 weeks and treated two groups of animals with varying doses of citicoline. The effects of citicoline on visual behavior response and white matter integrity were analyzed with optokinetics and MR neuroimaging.Methods
Animal preparation: 18 adult Long Evans received an intracameral injection of a crosslinking hydrogel4 that solidified to an optically clear hydrogel shortly after injection. The right eyes of all animals were injected and the left eye was uninjured and used as internal control. The 18 animals were divided into three groups: 1. Gel injection only (untreated, n=6), 2. low-dose citicoline (n=6), and 3. high-dose citicoline (n=6). The 12 animals in groups 2 and 3 received daily oral citicoline treatment (500mg/kg and 2500mg/kg, respectively) for 7 days prior to hydrogel injection, and every 48 hours for 14 days after hydrogel injection. IOP measurements: IOP was measured for 35 days after hydrogel injection with a handheld tonometer (ICare, Finland). A total of 18 measurements were averaged for each eye. Optokinetics: Visual behavior response was measured with an OptoMotry virtual reality system (CerebralMechanics) for 35 days after hydrogel injection5,6. MRI protocol: Diffusion tensor imaging was done at 35 days after hydrogel injection using a 9.4T MRI scanner. DTI was acquired with a fast-spin echo sequence with 12 diffusion gradient directions at b=1.0ms/μm2 and 2 non-diffusion-weighted images at b=0ms/μm2 (b0). Other imaging parameters included: slice thickness=1mm, acquisition matrix= 192x192 (zero-filled to 256×256), field of view=2.6x2.6cm2, number of repetitions=4, diffusion gradient duration time (δ)/diffusion gradient separation time (Δ)=5/17ms, ETL=8, and TR/TE= 2300/27.8ms. Slices were oriented orthogonal to the prechiasmatic optic nerves. MRI data analysis: Fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) were computed using DSIStudio. Regions of interest were manually drawn on the prechiasmatic optic nerve and the optic tract with reference to the rat brain atlas. DTI values were calculated as ratios between visual pathways projected from the IOP elevated eyes and the contralateral control eyes. Values were compared between untreated, low-dose citicoline, and high-dose citicoline groups using ANOVA and post-hoc multiple comparisons correction tests. Results are presented as average ± standard error of mean.Results
Intracameral injection of the cross-linking hydrogel to the right eyes significantly elevated the IOP in all animals for up to 35 days, with no statistical difference between the untreated, 500mg/kg citicoline treated, or 2500mg/kg citicoline treated animals (Fig. 1). The IOP of the left uninjured eyes remained unchanged over time. The visual acuity (VA) of the right untreated, low-dose citicoline, and high-dose citicoline animals decreased as early as at day 7. The VA of the untreated animals decreased further to day 14 and 35, while the VA of the citicoline treated animals stabilized at day 14, with the high dose animals having a significantly higher VA than the untreated animals (Fig. 2a). At the end experimental time point, untreated animals had 45% difference in VA between left and right eyes, whereas low and high dose citicoline treatment resulted in 25% and 10% VA difference, respectively (Figure 2b). The decreases in FA in the optic tract and AD in the optic nerve were significantly smaller in the 2500mg/kg animals compared to untreated animals (Fig. 3). Interestingly, both low and high citicoline dose resulted in less than 5% difference in FA between left and right optic nerves at day 35, as compared to 25% difference in the untreated group.Discussion and conclusions
Our results indicate oral citicoline administration ameliorates changes in the visual behavior response and white matter integrity after 5 weeks of IOP elevation in an experimental glaucoma model under both low and high citicoline doses. These results appear consistent with recent literature which suggests citicoline can slow down neurodegeneration and improve functional outcomes7. The fact that VA continued to improve at increasing citicoline dose while FA differences between injured and uninjured optic nerves remained similar at both doses, suggests that citicoline may act both on the optic nerve axons and beyond8.Acknowledgements
This work was supported by the National Institutes of Health P30-EY008098 and R01-EY028125 (Bethesda, Maryland); BrightFocus Foundation G2013077 and G2016030 (Clarksburg, Maryland), Eye and Ear Foundation (Pittsburgh, Pennsylvania); and Research to Prevent Blindness (New York, New York).References
[1] Susanna, R., Jr., De Moraes, C.G., Cioffi, G.A. & Ritch, R. Why Do People (Still) Go Blind from Glaucoma? Transl Vis Sci Technol 4, 1 (2015). [2] Grieb, P., Rejdak, R. Pharmacodynamics of citicoline relavant to the treatment of glaucoma. J Neurosci Res 14;67 (2002) [3] Parisi, V., Coppola, G., Centofanti, M., Oddone, F., Angrisani, AM., Ziccardi, L., Ricci, B., Quaranta, L., Manni, G. Evidence of the neuroprotective role of citicoline in glaucoma patients. Prog Brain Res (2008) [4] Ho LC, Sigal IA, Jan N-J, Yang X, Merwe Y van der, Yu Y, Chau Y, Leung CK, Conner IP, Jin T, Wu EX, Kim S-G, Wollstein G, Schuman JS, Chan KC. Non-invasive MRI Assessments of Tissue Microstructures and Macromolecules in the Eye upon Biomechanical or Biochemical Modulation. Sci Rep. 2016 Aug 26;6:srep32080. [5] Prusky, GT., Alam, NM., Beekman, S., Douglas, RM., Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. (2004) [6] Douglas, RM., Alam, NM., Silver, BD., McGill, TJ., Tschetter, WW., Prusky, GT. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci,5 (2005) [7] Skripuletz, T., Manzel A, Gropengießer K, Schäfer N, Gudi V, Singh V, Salinas Tejedor L, Jörg S, Hammer A, Voss E, Vulinovic F, Degen D, Wolf R, Lee DH, Pul R, Moharregh-Khiabani D, Baumgärtner W, Gold R, Linker RA, Stangel M. Pivotal role of choline metabolites in remyelination. Brain, 2015. [8] Chamoun M., Sergeeva EG., Henrich-Noack P., Jia S., Grigartzik L., Ma J., You Q., Huppé-Gourgues F., Sabel BA., Vaucher E..Cholinergic Potentiation of Restoration of Visual Function after Optic Nerve Damage in Rats. Neural Plast. 2017Figures
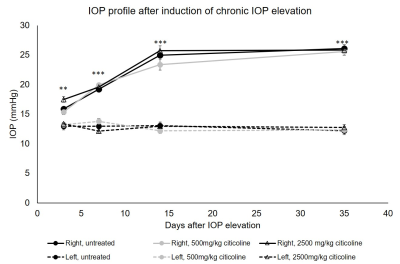
Figure 1. Intraocular pressure (IOP)
elevation in left and right eyes after unilateral chronic IOP elevation
induction with or without oral citicoline treatment. Post-hoc Tukey’s test
between left and right eyes of the untreated, 500mg/kg citicoline treated, and
2500mg/kg citicoline treated groups (**p<0.01, ***p<0.001). No apparent
IOP difference was found in the right eyes between untreated, 500mg/kg
citicoline treated, and 2500mg/kg citicoline treated groups.
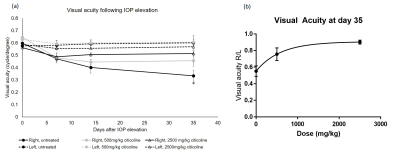
Figure 2 (a). Visual acuity of left and
right eyes after unilateral acute IOP elevation induction with or without
citicoline treatment. Post-hoc Tukey’s test between right eyes of the
untreated, 500mg/kg citicoline treated, and 2500mg/kg citicoline treated
groups. Post-hoc Tukey’s test in the right eye between untreated and 2500mg/kg
treated groups (*p<0.05). (b) Dose-dependent response to visual acuity in
the right eye relative to the left eye at 35 days after hydrogel injection to
the right eye.
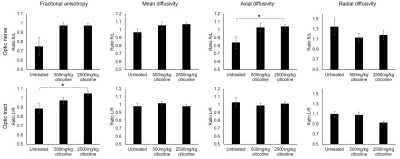
Figure 3. Diffusion tensor imaging in the
optic nerve and optic tract 35 days after IOP elevation induction with or
without citicoline treatment. Values presented as a ratio between visual
pathways projected from injured and control visual pathways. Post-hoc Tukey’s test between groups
(*p<0.05).