3610
Abnormal Gray Matter Structural networks in Idiopathic Normal Pressure Hydrocephalus1Radiology Deparment, Shanghai Chest Hospital, Shanghai, China, 2Radiology Department, Huashan Hospital of Fudan University, Shanghai, China, 3Radiology Department, Shanghai Chest Hospital, Shanghai, China
Synopsis
Idiopathic normal pressure hydrocephalus (iNPH) is a neurological disorder, the structural networks changes were never studied. We examined changes in gray matter structural network of patients with iNPH comparing with normal elderly people. Global network modularity was significantly larger in the iNPH network compared with the NC network (P<0.05). Eight nodes with significantly decreased betweenness were found in right frontal, temporal, insula lobe and right posterior cingulate region of iNPH network, while only one node was detected with significantly larger betweenness. Hubs of the iNPH network were mostly located in temporal areas and limbic lobe, while hubs of NC network were mainly located in frontal areas. We found some abnormalities in gray matter structural network that may relate to the occurrence of iNPH.
INTRODUCTION
Idiopathic normal pressure hydrocephalus (iNPH) is a neurological disorder characterized by gait disturbance, cognitive impairment, and incontinence. There is little known about the pathogenesis of iNPH as far to now. Many chronic brain disorders with cognitive, emotional, perceptual and motor symptoms are associated with abnormalities of brain network organization1. However, few study have examined the brain network changes of iNPH patients. Alteration in default mode network of iNPH patients has been detected using resting-state functional MRI2. The structural network of iNPH patients has never been examined. The authors aimed to investigate alterations in the gray matter structural network of patients with iNPH comparing with normal elderly people.METHODS
Total of 33 patients fulfilled the diagnosis of possible iNPH according to the International iNPH guideline and 33 age- and gender- matched volunteers were recruited and received MR scanning. The structural network was reconstructed using covariance between regional gray matter volumes extracted from three dimensional T1 weighted imaging of 29 possible iNPH patients and 30 demographically similar normal-control (NC) participants. The networks of the iNPH group and NC group were examined using graph theory and compared with each other. Global network measures were compared in a range of network density (Dmin: .1: .02: .5). Global network analysis, reginal network analysis and between-group comparison were performed. FDA and AUC analysis on global network measures was also conducted to ensure the iNPH-NC differences were not driven by differences in correlation strengths in regional gray matter volumes and make the analysis less sensitive to thresholding.RESULTS
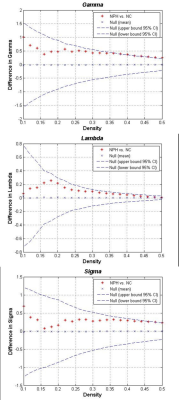
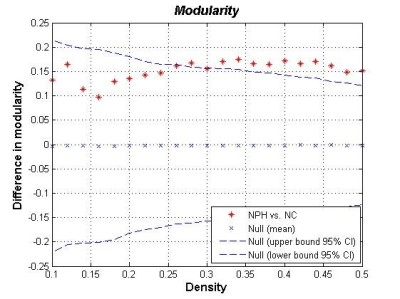
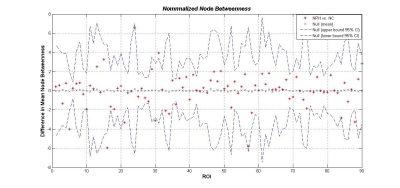
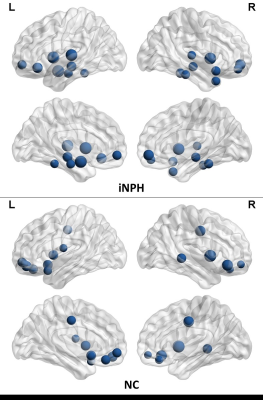
The networks of both iNPH group and NC group followed a small-world organization across a range of densities. While small-world measures were not significantly different between iNPH group and NC group network (P>0.05), even though normalized clustering and small-world index of the two networks were significantly different in some network density. Global network modularity was significantly larger in the iNPH network compared with the NC network at density of 0.28 and densities range from 0.32 to 0.5 (Fig.4). The FDA and AUC analysis also revealed a significantly lager modularity in iNPH network (P= 0.043 and 0.042, respectively). Regional network analysis was conducted in the minimum density under which all nodes were fully connected. Eight nodes with significantly decreased betweenness were found in right frontal, temporal, insula lobe and right posterior cingulate region of iNPH network, while only one node was detected with significantly larger betweenness. Hubs of the iNPH network were mostly located in temporal areas and limbic lobe, while hubs of NC network were mainly located in frontal areas.DISCUSSION
The present study examined the small-world characteristic of GM volume correlation networks of iNPH patients and NC group. The both networks have the properties with cohesive neighborhoods and short path length between regions3, 4. INPH network showed higher clustering coefficient, short path length and small-world index, the between group differences were not statistically significant. He et.al reported a similar but more significant difference about small world characteristic in Alzheimer’s disease and suspected that the altered coordinated patterns of cortical morphology may related to the cognitive impairment5. More obvious, is the different of modularity between the iNPH network and NC network. INPH network represents a treatable form of dementia. It has been reported that brain network modularity may be a valuable biomarker that can inform the implementation of cognitive interventions6. Diffusion tensor imaging (DTI) method can reveal subtle injures such as axonal loss and gliosis in white matter. LENFELDT N et.al reported lesions in anterior frontal white matter using this method, and suspected this change was related to motor symptoms in INPH7. Moreover, based on voxel-wise analysis of DTI, various patterns of white matter changes were detected in more border regions, such as corpus callosum, periventricular white matter, internal capsule and so on8. This may explain that several nodes of iNPH network showed significantly decreased node betweenness, while only one node was detected increased.CONCLUSION
As the first study of structural network in iNPH patients, we found some particular abnormalities may relate to the occurrence of this disease. And network modularity changes in this possible iNPH group was detected and deserve to be studied further. Network connectivity study using DTI-based tractography method or BOLD-MRI to detect the network measures of each subject is promising to clarify the pathological mechanism of iNPH.Acknowledgements
This work was supported by a grant from Population and FamilyPlanning Commission of Pudong New Area, Shanghai Municipality,China (PW2012D-9)
References
- Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci 2012; 13(5):336-49.
- Khoo HM, Kishima H, Tani N, et al. Default mode network connectivity in patients with idiopathic normal pressure hydrocephalus. Journal of Neurosurgery 2016; 124(2):350-358.
- He Y, Chen Z, Gong G, Evans A. Neuronal networks in Alzheimer's disease. Neuroscientist 2009; 15(4):333-50.
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009; 10(3):186-98.
- He Y, Chen Z, Evans A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer's disease. J Neurosci 2008; 28(18):4756-66.
- Arnemann KL, Chen AJ, Novakovic-Agopian T, et al. Functional brain network modularity predicts response to cognitive training after brain injury. Neurology 2015; 84(15):1568-74.
- Lenfeldt N, Larsson A, Nyberg L, et al. Diffusion tensor imaging reveals supplementary lesions to frontal white matter in idiopathic normal pressure hydrocephalus. Neurosurgery 2011; 68(6):1586-93; discussion 1593.
- Hattori T, Ito K, Aoki S, et al. White matter alteration in idiopathic normal pressure hydrocephalus: tract-based spatial statistics study. AJNR Am J Neuroradiol 2012; 33(1):97-103.
Figures