3608
Iron accumulation in the striatonigral pathway of patients with Fabry Disease1Scienze Biomediche Avanzate, Università degli Studi di Napoli "Federico II", Napoli, Italy, 2University “Federico II”, Napoli, Italy, 3IRCCS SDN, Napoli, Italy, 4Institute of Biostructure and Bioimaging, National Research Council, Napoli, Italy
Synopsis
In Fabry Disease (FD) patients, compared to healthy controls, a significant increase in magnetic susceptibility has been observed in the substantia nigra and in the striatum, associated to a significant volume loss limited to the single substantia nigra. These findings probably reflect neurodegenerative phenomena due to pathological iron deposition in these particular extrapyramidal relay stations. This evidence supports the current hypothesis of a permeative cerebral involvement in FD that goes further the pure cerebrovascular association, thus shedding new light on this condition.
Introduction
Fabry Disease (FD) is a rare disorder due to insufficient enzymatic activity of alpha-galactosidase A, with progressive lysosomal deposition of uncatabolized lipids (1). Recent evidences suggested that prodromal signs of neurodegeneration affecting motor functions could be present in FD (2). This motor impairment is mainly expressed as a sub-clinical extrapyramidal syndrome with prevalence of motor slowing and postural instability over rigidity and tremor (2). Quantitative Susceptibility Mapping (QSM) allows for a consistent and quantitative evaluation of pathologic tissue changes, specifically iron accumulation (3), previously described in other disorders affecting extrapyramidal system, such as Parkinson Disease (PD) (4-6). To date no study has been performed to investigate iron accumulation in striatonigral structures in FD, seeming to share some pathological phenomena with early PD (7). Aim of this study was to investigate the presence of abnormal iron accumulation in striatonigral relay stations of FD patients, to further elucidate pathophysiological mechanisms of damage in FD.Methods
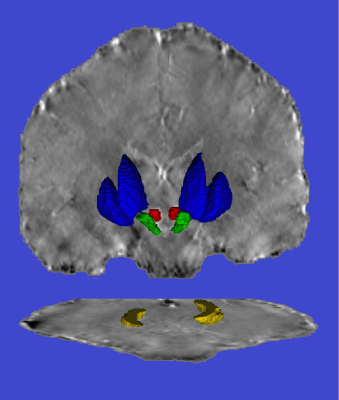
30 FD patients and 37 healthy controls (HC) matched for age and sex were enrolled. All subjects underwent 3T MRI scan including a Magnetization Prepared Rapid Acquisition Gradient Echo sequence (MPRAGE) and an unenhanced 3D Dual-Echo spoiled Gradient-Echo sequence (FLASH) used for Quantitative Susceptibiliy Mapping (QSM). Striatum segmentation was computed on the MPRAGE as the union of the masks of globus pallidus, putamen and caudate nuclei automatically obtained using the FIRST routine (FMRIB’s Integrated Registration Segmentation Toolkit http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST, FSL package). The MPRAGE image was affine registered to the first echo of the FLASH, and registration matrix was used to register the segmentation masks in the QSM space. To avoid possible biases, the segmentation masks were eroded with a spherical structuring element with radius of 1mm; an expert neuroradiologist confirmed the quality of the automated process. The other extrapyramidal structures (red nuclei –RN–, substantia nigra –SN–, dentate nuclei –DN–) were manually segmented, using two irregular bilateral Region Of Interest (ROIs) hand-drawn in consensus by three experienced neuroradiologists on each axial slice of the QSM containing the above-mentioned structures. An example of the result of the segmentation is shown in Figure 1. Group differences were tested using a generalized linear model, including age and sex as covariates.Results
FD patients showed significantly higher QSM values in the SN (189.07±16.36 ppb for FD patients compared to 176.68±9.73 ppb for HC, p=0.0002) and in the striatum (73.3 ± 13.9 ppb vs 66.2 ± 12.3 ppb for FD and HC, respectively; p=0.005). Furthermore, FD patients had a significant reduction in SN volume compared to HC (1153.56±407.47 mm3 for FD patients vs 1509.32±229.01 mm3 for HC, p<10-5). No significant difference was found in RN and DN, neither in terms of susceptibility nor in terms of volume loss.Discussion
Our findings expand recent knowledge about alterations affecting the extrapyramidal pathway in FD patients, showing a predominant involvement of the SN among the others investigated structures both in terms of susceptibility increase and volumetric reduction. Current hypothesis of CNS involvement in FD is that a combination of reduced local perfusion and intracellular accumulation of uncatabolized substrates could result in the generation of toxic reactive oxygen species contributing to progressive neurodegeneration and atrophy of neural cells (8,9), similarly to what happens in degenerative disorders affecting extrapyramidal pathway such as PD. Taking into account some reported cases of comorbidity between PD and FD (10,11), and given the mild motor impairment recently described in FD as well as its similarities with early PD (akinetic/rigidity-dominant PD phenotype specifically) (2,7), we decided to investigate the motor system in FD in terms of brain iron accumulation (12). A significant susceptibility increase in FD patients was observed in the two major stations of the extrapyramidal system, the SN and the striatum, somehow similarly to what previously described in PD (4-6). This apparently selective involvement, with relative sparing of other extrapyramidal examined structures allows us to further speculate that FD patients could share some physiopathological modification with PD, with particular reference to the akinetic/rigidity-dominant PD phenotype (13). When testing for consensual volume loss, another hallmark of neurodegeneration in PD (14), only SN showed a volumetric reduction in FD patients; a possible explanation to the relative sparing of striatum could be researched in the different susceptibility to degenerative processes of different extrapyramidal structures (15-20).Conclusion
FD patients show higher QSM values in SN and striatum, with an associated significant volume loss limited to the SN. These changes add further evidences and contribute to understand the physiopathological bases of extrapyramidal involvement in FD.Acknowledgements
No acknowledgement found.References
1. Germain DP. Fabry disease. Orphanet journal of rare diseases. 2010 Nov 22;5:30.
2. Lohle M, Hughes D, Milligan A, et al. Clinical prodromes of neurodegeneration in Anderson-Fabry disease. Neurology. 2015 Apr 07;84(14):1454-1464.
3. Palma G, Tedeschi E, Borrelli P, et al. A Novel Multiparametric Approach to 3D Quantitative MRI of the Brain. PLoS One. 2015;10(8):e0134963.
4. Murakami Y, Kakeda S, Watanabe K, et al. Usefulness of quantitative susceptibility mapping for the diagnosis of Parkinson disease. AJNR Am J Neuroradiol. 2015 Jun;36(6):1102-1108.
5. Azuma M, Hirai T, Yamada K, et al. Lateral Asymmetry and Spatial Difference of Iron Deposition in the Substantia Nigra of Patients with Parkinson Disease Measured with Quantitative Susceptibility Mapping. AJNR Am J Neuroradiol. 2016 May;37(5):782-788.
6. Langkammer C, Pirpamer L, Seiler S, et al. Quantitative Susceptibility Mapping in Parkinson's Disease. PLoS One. 2016;11(9):e0162460.
7. Cocozza S, Pisani A, Olivo G, et al. Alterations of functional connectivity of the motor cortex in Fabry disease: An RS-fMRI study. Neurology. 2017 May 09;88(19):1822-1829.
8. Zarate YA, Hopkin RJ. Fabry's disease. Lancet. 2008 Oct 18;372(9647):1427-1435.
9. Paris I, Martinez-Alvarado P, Cárdenas S, Perez-Pastene C, Graumann R, Fuentes P, Olea-Azar C, Caviedes P, Segura-Aguilar J. Dopamine-dependent iron toxicity in cells derived from rat hypothalamus. Chem Res Toxicol. 2005 Mar;18(3):415-9.
10. Borsini W, Giuliacci G, Torricelli F, et al. Anderson-Fabry disease with cerebrovascular complications in two Italian families. Neurol Sci. 2002 Jun;23(2):49-53.
11. Buechner S, De Cristofaro MT, Ramat S, Borsini W. Parkinsonism and Anderson Fabry's disease: a case report. Mov Disord. 2006 Jan;21(1):103-107.
12. Acosta-Cabronero J, Cardenas-Blanco A, Betts MJ, et al. The whole-brain pattern of magnetic susceptibility perturbations in Parkinson's disease. Brain. 2017 Jan;140(Pt 1):118-131.
13. He N, Huang P, Ling H, et al. Dentate nucleus iron deposition is a potential biomarker for tremor-dominant Parkinson's disease. NMR Biomed. 2017 Apr;30(4).
14. Dickson DW. Neuropathology of Parkinson disease. Parkinsonism Relat Disord. 2017 Aug 01.
15. Menke RA, Jbabdi S, Miller KL, Matthews PM, Zarei M. Connectivity-based segmentation of the substantia nigra in human and its implications in Parkinson's disease. Neuroimage. 2010 Oct 01;52(4):1175-1180.
16. Blazejewska AI, Schwarz ST, Pitiot A, et al. Visualization of nigrosome 1 and its loss in PD: pathoanatomical correlation and in vivo 7 T MRI. Neurology. 2013 Aug 06;81(6):534-540.
17. Camlidag I, Kocabicak E, Sahin B, et al. Volumetric analysis of the subthalamic and red nuclei based on magnetic resonance imaging in patients with Parkinson's disease. Int J Neurosci. 2014 Apr;124(4):291-295.
18. Menke RA, Scholz J, Miller KL, et al. MRI characteristics of the substantia nigra in Parkinson's disease: a combined quantitative T1 and DTI study. Neuroimage. 2009 Aug 15;47(2):435-441.
19. Schwarz ST, Afzal M, Morgan PS, et al. The 'swallow tail' appearance of the healthy nigrosome - a new accurate test of Parkinson's disease: a case-control and retrospective cross-sectional MRI study at 3T. PLoS One. 2014;9(4):e93814.
20. Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003 Mar-Apr;24(2):197-211.