3601
Disrupted topological organization of brain structural network associated with prior overt hepatic encephalopathy in cirrhotic patients1Fujian Medical University Union Hospital, Fuzhou, China
Synopsis
Disrupted topological organization of brain structural network associated with prior overt hepatic encephalopathy
Introduction
Overt hepatic encephalopathy (OHE) is a serious complication of cirrhosis that significantly reduces patients’ survival [1-3]. The extant findings suggest that the occurrence of OHE leads to a less-than-full recovery of brain function. Both cross-sectional and longitudinal studies have demonstrated that once patients have had a bout of OHE, they would develop persistent cognitive impairment (e.g., impaired working memory, response inhibition, and learning capacity), even after resolution [4-6]. However, the underlying pathogenesis of the persistent brain function changes associated with prior OHE is not well understood.
Several anatomic neuroimaging studies have revealed the potential effects of prior OHE on brain morphology in vivo [7] [8] [9]. Despite the existing findings suggesting that prior OHE-related cognitive decline may result from regional brain anatomic changes, we still lack a comprehensive understanding of the effect of prior OHE on the whole-brain structural network that can fully explain the connectivity between the distinct regions. There is increasing evidence suggesting that the human brain is essentially a complex patchwork of interconnected regions and should be considered as a large-scale integrated network (i.e. connectome) in both functional and structural domain [10-12]. The network approach has become increasingly useful for understanding human cognitive function and providing valuable insights into the fundamental mechanisms underlying various neuropsychological disorders [11]. Therefore, it may be appropriate to correlate cognitive dysfunctions associated with prior OHE to changes in brain network properties, rather than specific brain regions, in order to explore the relevant neural mechanisms more comprehensively.
Diffusion tensor imaging (DTI) is a powerful imaging technique that can be used to map white matter structural connectivity in vivo through tractography methods. Examining the alterations in the topological properties of the white matter structural network is very helpful for enhancing our understanding about the mechanisms behind neuropsychiatric diseases [12; 13]. In this study, the DTI-based structural brain connectome analysis was performed in the cirrhotic patients with and without prior OHE. The brain network properties are examined by graph theory-based analyses and compared across groups to assess the persistent effects of prior OHE on topological organization of brain structural network.
Methods
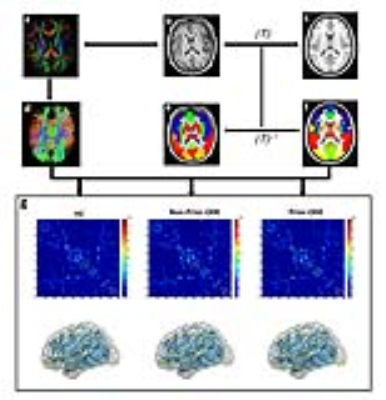
Seventeen cirrhotic patients with prior OHE (Prior-OHE), 18 cirrhotic patients without prior OHE (Non-Prior-OHE), and 18 healthy controls (HC) underwent diffusion tensor imaging. Neurocognitive functioning was assessed with Psychometric Hepatic Encephalopathy Score (PHES). Using a probabilistic fiber tracking approach, whole-brain structural network was depicted as a connectivity matrix of 90 regions (derived from Automated Anatomic Labeling atlas). Graph theory-based analyses were performed to analyze topological properties of brain network (Figure 1).Results
The Analysis of Variance showed significant group effects on several topological properties, including network strength, global efficiency, and local efficiency (Figure 2). A progressive decrease trend for these metrics was found from Non-Prior-OHE to Prior-OHE, compared with HC. Among three groups, the regions with altered nodal efficiency were mainly distributed in the frontal and occipital cortices, paralimbic system, and subcortical regions (Figure 3). The topological metrics, such as network strength and global efficiency, were correlated with PHES among cirrhotic patients (Figures 4-5).Discussion
Our results are consistent with previous findings in functional MRI studies, which have revealed the disrupted topological organization of the whole-brain network in cirrhotic patients during OHE episodes, indicated by lower clustering coefficiency, decreased network connectivity strength, and impaired modularity [14]. The OHE episode could also induce reduction of the local and global topological efficiencies of the brain functional network [15]. Our results suggest that the functional network abnormalities that occur in OHE may be based on the altered topological organization of the structural WM network. Meanwhile, our results demonstrated that the impairment of brain network could persist after the resolution of OHE, since a deteriorating trend was observed for network measures from Non-Prior-OHE to Prior-OHE. This is in agreement with previous findings by functional network analysis [16], in which more significant disruption of functional connectivity in DMN connectivity was found in Prior-OHE, relative to Non-Prior-OHE. Of note, the more deteriorative topological organization of the brain network may be an important reason for the higher risk of cognitive impairments in Prior-OHE patients, considering the correlation between structural network metrics and cognitive performance.
In addition, consistent with the regional brain anatomic changes reported in previous studies, the distribution of brain regions with altered nodal efficiency was mainly located in the frontal and occipital cortices, cingulate gyrus, and subcortical regions in Prior-OHE patients.
Conclusion
The cirrhotic patients developed structural brain connectome alterations, which is aggravated by prior OHE episode. Disrupted topological organization of brain structural network may account for cognitive impairments related to prior OHE.Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (No. 81501450) and a project funded by the China Postdoctoral Science Foundation.References
1. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761.
2. Stepanova M, Mishra A, Venkatesan C, et al. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10:1034-1041 e1.
3. Bustamante J, Rimola A, Ventura PJ, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30:890-895.
4. Bajaj JS, Schubert CM, Heuman DM, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138:2332-2340.
5. Umapathy S, Dhiman RK, Grover S, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Am J Gastroenterol. 2014;109:1011-1019.
6. Riggio O, Ridola L, Pasquale C, et al. Evidence of persistent cognitive impairment after resolution of overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2011;9:181-183.
7. Guevara M, Baccaro ME, Gomez-Anson B, et al. Cerebral magnetic resonance imaging reveals marked abnormalities of brain tissue density in patients with cirrhosis without overt hepatic encephalopathy. J Hepatol. 2011;55:564-573.
8. Chen HJ, Wang Y, Zhu XQ, et al. White matter abnormalities correlate with neurocognitive performance in patients with HBV-related cirrhosis. J Neurol Sci. 2012;321:65-72.
9. Butterworth R. Neuronal cell death in hepatic encephalopathy. Metab Brain Dis. 2007;22:309-320.
10. Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277-290.
11. Barkhof F, Haller S, Rombouts SA. Resting-state functional MR imaging: a new window to the brain. Radiology. 2014;272:29-49. 12. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186-198.
13. Griffa A, Baumann PS, Thiran JP, et al. Structural connectomics in brain diseases. Neuroimage. 2013;80:515-526.
14. Jao T, Schroter M, Chen CL, et al. Functional brain network changes associated with clinical and biochemical measures of the severity of hepatic encephalopathy. Neuroimage. 2015;122:332-344.
15. Hsu TW, Wu CW, Cheng YF, et al. Impaired small-world network efficiency and dynamic functional distribution in patients with cirrhosis. PLoS One. 2012;7:e35266.
16. Chen HJ, Jiao Y, Zhu XQ, et al. Brain dysfunction primarily related to previous overt hepatic encephalopathy compared with minimal hepatic encephalopathy: resting-state functional MR imaging demonstration. Radiology. 2013;266:261-270.
Figures