3600
Cellular MRI reveals altered brain arrest of genetically-engineered reporter-expressing metastatic breast cancer cellsKatie Parkins1, Veronica Dubois1, Suzy Wong1, Amanda Hamilton2, Paula Foster1, and John Ronald1
1Medical Biophysics, Western University, London, ON, Canada, 2Western University, London, ON, Canada
Synopsis
While BLI is very complementary to MRI in evaluating the fate of many different cell populations in vivo, including cancer cells, some considerations for the use of BLI have been reported including an increase in tumor growth variation as well as a change in metastatic pattern following luciferase tagging. The objective of this work was to use cellular and anatomical MRI to characterize the in vivo growth patterns of naive and lentiviral-engineered brain-seeking breast cancer cell lines co-expressing fluorescent and bioluminescent reporters in the mouse brain.
Introduction
The majority of cancer patients die due to metastatic disease. Noninvasive imaging of the metastatic spread of cancer in preclinical models can provide important information on the steps involved, as well as better evaluation of anti-metastatic treatments. Our group has been exploring the use of anatomical MRI, cellular MRI and bioluminescence imaging (BLI) for sensitive and improved monitoring of experimental breast cancer brain metastasis in mice1. By using these complementary technologies, we have acquired valuable measurements of viable cancer cell arrest in the brain after systemic administration, the clearance and/or retention of these cells thereafter, as well as the growth and change in viability of these cells over time. While BLI is very useful in measuring the viability of cancer cells, some considerations have been reported using luciferase-tagged cells such as an increased tumor volume variation across animals, changes in the pattern of metastasis2, and inhibition of in vivo tumor growth at high luciferase expression levels3. Conversely, many groups have shown that, compared to naïve cells, luciferase-tagging and BLI does not alter cancer cell proliferation or in vivo tumor distribution, and BLI is widely used in tracking non-cancerous cells without obvious effects. Our objective was to use cellular and anatomical MRI to evaluate in vivo growth patterns of naive and luciferase-expressing brain-seeking breast cancer cell lines in the mouse brain.Methods
Murine (4T1BR5) and human (MDA-MB-231BR-eGFP) brain-seeking breast cancer cells were engineered with lentiviral vectors expressing luciferase (Luc) and eGFP. 4T1 Model: BALB/c mice (n=16) received an intracardiac injection of 2x104 iron-labeled 4T1BR5 or 4T1BR5-Luc cells. 231 Model: Nu/nu mice (n=8) received an intracardiac injection of 1.5x105 iron-labeled MDA-MB-231BR-eGFP or MDA-MB-231BR-eGFP-Luc cells. Cellular MRI was performed at day 0 on a 3T scanner using customized gradient and solenoidal RF coils and the iron-sensitive bSSFP sequence to measure whole-brain single cell arrest (iron-induced MR signal voids). MRI was also performed using the same sequence to measure the number and volume of metastases at endpoint (day 14 for 4T1 mice, day 28 for 231 mice). BLI was performed using an optical imaging scanner only on mice receiving Luc+ cells to assess viable cell arrest at day 0 and viable cancer cells in the brain at endpoint.Results
4T1 Model: Iron labeled cells were visualized in brain MR images as discrete signal voids and the number of voids on day 0 was significantly higher in mice receiving naïve 4T1BR5 cells compared to mice receiving 4T1BR5-Luc cells (p<0.001; Figure1A/C). Brain BLI signal was detectable at day 0 in mice that received 4T1BR5-Luc cells (Figure1B). At day 14, hyperintense metastases were apparent in MR images and BLI signal could be detected in the brain and body of mice that received 4T1BR5-Luc cells (Figure2A/B). Mice that received naïve 4T1BR5 cells had significantly higher brain tumor burden and brain metastases than mice that received 4T1BR5-Luc cells (p<0.001; Figure2C/D). 231 Model: The percent of black pixels (semi-automatic quantification of discrete voids)1 on day 0 was significantly higher in mice receiving 231BR-eGFP cells compared to mice receiving 231BR-eGFP-Luc cells (p<0.001; Figure3A/C). BLI signal was detectable at day 0 (Figure3B), and could be detected in brain and other areas at endpoint in mice receiving 231BR-eGFP-Luc cells (Figure4C). Mice that received naïve 231BR-eGFP cells had significantly higher brain tumor burden and brain metastases than mice that received 231BR-eGFP-Luc cells (p<0.05; Figure4C/D).Discussion
An important step in the metastatic cascade is the initial arrest of circulating tumor cells. Past studies have found that engineering cancer cells with reporter genes can have an effect on tumor growth2-4, which has mostly been attributed to decreased cellular proliferation of cells in vivo. Our results point to decreased arrest of engineered cancer cells as another possible explanation for differences in end tumor burden. Altered arrest could be the result of several possibilities including use of lentiviral vectors that integrate into the genome causing altered gene expression, selective growth and selection of a subset of cells during the engineering process, or directly due to the expression of luciferase and other transgenes.Conclusion
For the first time, we have applied cellular and molecular imaging tools to characterize in vivo growth differences between naïve and engineered cell lines in two well-established mouse models of breast cancer metastasis. By employing cellular MRI we have demonstrated that cell engineering has a significant effect on cell arrest in the brain. This indicates engineering cancer cells with reporter genes may alter their tropism towards particular organs, and care should be taken when using reporter genes for in vivo cellular imaging of cancerous, and possibly non-cancerous, cell populations.Acknowledgements
No acknowledgement found.References
- Parkins, K. M., Hamilton, A. M., Makela, A. V., Chen, Y., Foster, P. J., & Ronald, J. A. (2016). A multimodality imaging model to track viable breast cancer cells from single arrest to metastasis in the mouse brain. Sci. Rep., 6, 35889.
- Milsom, C. C., Lee, C. R., Hackl, C., Man, S., & Kerbel, R. S. (2013). Differential post-surgical metastasis and survival in SCID, NOD-SCID and NOD-SCID-IL-2Rγnull mice with parental and subline variants of human breast cancer: implications for host defense mechanisms regulating metastasis. PloS one, 8(8), e71270.
- Brutkiewicz, S., Mendonca, M., Stantz, K., Comerford, K., Bigsby, R., Hutchins, G., ... & Harrington, M. (2007). The expression level of luciferase within tumour cells can alter tumour growth upon in vivo bioluminescence imaging. Luminescence, 22(3), 221-228.
- Clark, A. J., Safaee, M., Oh, T., Ivan, M. E., Parimi, V., Hashizume, R., ... & Parsa, A. T. (2014). Stable luciferase expression does not alter immunologic or in vivo growth properties of GL261 murine glioma cells. Journal of translational medicine, 12(1), 345.
Figures
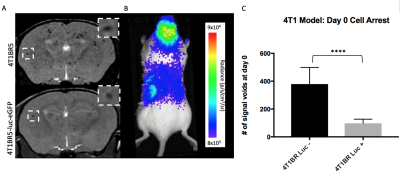
Figure 1: Iron labeled cells were visualized in
brain MR images as discrete signal voids on day 0 (A). Brain BLI signal was
also detectable at day 0 in mice that received luciferase expressing cells (B).
The number of voids on day 0 was significantly higher (p<0.001) in mice
receiving naïve 4T1BR5 cells compared to mice receiving luciferase-expressing
4T1BR5 cells (C).
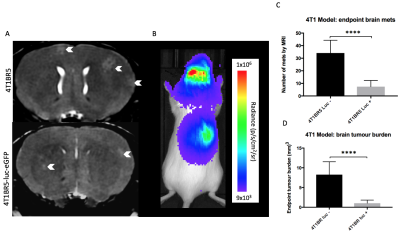
Figure 2: At day 14, brain metastases appeared as
regions of hyperintensity by MRI (A). BLI signal could be detected
in the brain and other areas of the body in mice that received luciferase
expressing cells (B). Mice that received naïve 4T1BR5 cells had significantly
more (p<0.001) brain metastases (C) and brain tumor burden (D) than mice that received
luciferase-expressing 4T1BR5 cells.
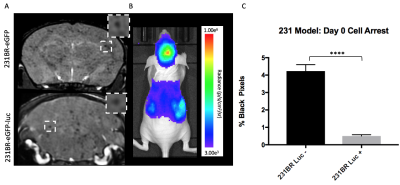
Figure
3: Iron-labeled cells were visualized as discrete signal voids in brain MR
images on day 0 (A). Brain BLI signal was also detectable at
day 0 in mice that received luciferase
tagged cells (B). The percent of black pixels
(representing discrete signal voids) on day 0 was significantly higher
(p<0.001) in mice receiving 231BR-eGFP cells compared to mice receiving
luciferase-expressing 231BR-eGFP cells (C).
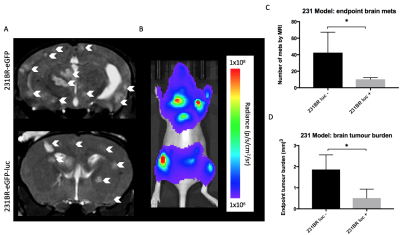
Figure 4: At day 28, brain metastases appeared as
regions of hyperintensity by MRI (A). BLI signal could be detected
in the brain and other areas of the body in mice that received luciferase
expressing cells (B). Mice that received MDA-MB-231BR-eGFP cells
had significantly more (p<0.05) brain metastases (C) and brain tumor burden (D) than
mice that received MDA-MB-231BR-eGFP cells.