3599
Novel tracking method for cellular patterns obtained by Laser-Assisted Bioprinting using Magnetic Resonance Imaging1Biotis, INSERM-Univ.Bordeaux, Bordeaux, France, 2CRMSB UMR5536, CNRS-Univ.Bordeaux, Bordeaux, France
Synopsis
One major issue of in situ bioprinting is related to cell pattern imaging in vivo. Magnetic Resonance Imaging (MRI) associated with Micron-sized superparamagnetic Iron Oxide (MPIO) particles constitutes a non-invasive method for tracking cells in vivo. In this study, optimal MPIO concentrations for tracking bioprinted cells were determined. Cell densities of patterns and MRI signals were correlated. MRI was used to track cell patterns in vitro and post-mortem, after in situ bioprinting onto a mouse calvaria defect. Results indicate that MRI combined with MPIO cell labeling is a valuable technique to track bioprinted cells with sufficient precision.
INTRODUCTION
Major progresses in the field of Tissue Engineering allowed to control the three-dimensional organization of the scaffold and cells to reach in vivo applications. Beside biomaterial printing, Laser-Assisted cell Bioprinting (LAB) has grown considerably last years due to its rapidity, reproducibility, precision and ability to print viable cells1. This technology has been considered recently for in vivo applications in regenerative medicine2,3. One major issue of in situ bioprinting onto deep tissues is related to cell pattern imaging and follow-up in vivo. Magnetic Resonance Imaging (MRI) associated with Micron-sized superparamagnetic Iron Oxide (MPIO) particles, as contrast agents, constitutes a non-invasive method for tracking cells in vivo4–6. Despite the biocompatibility, low toxicity and high sensitivity of this method, no studies have currently considered cell labeling with MPIO in order to follow cell patterns organized by bioprinting technologies. The aim of this study was to assess the methodology of tracking MPIO-labeled cells using MRI after organizing them by Laser-Assisted Bioprinting.METHODS
The LAB workstation used in this study was previously described7. A dedicated software was used to control pattern design and substrate position. Stem cells from Apical Papilla (SCAPs) were used. In order to detect the cell patterns upon in vitro and post-mortem printing, SCAPs were transduced with GFP- or TdTomato- expressing lentiviral vectors. SCAPs were also labeled with different concentrations of MPIO (1:1000, 1:500, 1:200, 1:100). The laser focused on the gold layer induces the transfer of droplets of cells onto a collagen-coated substrate according to the selected pattern. Three geometries of pattern were designed: successive lines, disk and ring. Then, a “ring” pattern was printed onto a mouse bone calvaria defect with the optimal printing conditions determined in vitro. MRI experiments were performed on 4.7T Bruker Biospec system. A dedicated device was made for in vitro studies. It was composed of a circular surface coil (20mm diameter, DotyScientific) for signal excitation and reception, 2 vials fulfilled with water placed below the coil for MR system adjustment and a thin plate above to precisely installed Petry dishes containing bio-printed cells. Accuracy of the patterns was analyzed in vitro by confocal microscopy and in vivo by fluorescence microscopy. Post-mortem MRI was performed on the same MRI system with a 4-channels phased array coil dedicated to mouse brain. A T2*-w gradient echo sequence was used with following parameters: TE/TR=3.7/8ms; FOV=25x18x6mm; Matrix=256x192x64; resolution=97x94x94μm; Numbers of Excitations=1 or 256; Acquisition time: 6min33s or 7h respectively for in vitro and post-mortem studies.RESULTS
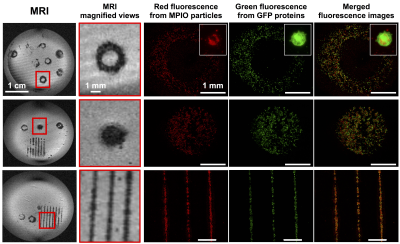
Pattern accuracy was observed in vitro by comparing MRI and confocal microscopy images (Figure 1). MRI signal intensity significantly increased when the MPIO concentration was higher than 1:500. A MPIO concentration of 1:200 was chosen as the optimal concentration since it led to a good sensitivity of detection on the MR images and to the best accurate reproduction of printed patterns. As the cell density of bioprinted patterns increased, the area covered by the MPIO-labeled cells increased on the MR images. A linear correlation was found between the area of the hypo-intense signal and the amount of bioprinted cells (R2=0.9991). After a 7-day in vitro follow-up of MPIO-labeled MSC samples, SCAPs were still easily detectable on the MR images. The area covered by the MSC and detected by MRI increased over time. Based on the printing geometries used in vitro, the “ring” pattern, was used for in situ bioprinting onto a bone calvaria defect in mouse. After an observation by fluorescence microscopy, a ring of lack of signal within the defect was detected post-mortem on the MR images, that could correspond to MPIO-labeled printed cells.DISCUSSION
The results presented here suggest the feasibility to use MRI and MPIO-labeled cells to track cell patterns organized by LAB in vitro and in situ onto mouse calvaria bone defect. In situ Laser-Assisted Bioprinting could constitute a new therapeutic approach allowing for precise organization of cells or biomaterials to implant at a micron scale. MRI, used as a method for tracking bioprinted cell patterns, would allow to control the procedure or evolution of in situ bio-printed tissue onto a patient.CONCLUSION
Here, MRI was used to precisely track MPIO-labeled SCAPs in vitro and post-mortem after patterning by LAB technology. Adjusting laser parameters and MPIO concentrations was performed to optimize the MRI detection of patterns.Acknowledgements
No acknowledgement found.References
1. Schiele, N. R. et al. Laser-based direct-write techniques for cell printing. Biofabrication 2, 032001 (2010).
2. Keriquel, V. et al. In vivo bioprinting for computer- and robotic-assisted medical intervention: preliminary study in mice. Biofabrication 2, 014101 (2010).
3. Keriquel, V. et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 7, (2017).
4. Ittrich, H., Peldschus, K., Raabe, N., Kaul, M. & Adam, G. Superparamagnetic iron oxide nanoparticles in biomedicine: applications and developments in diagnostics and therapy. ROFO. Fortschr. Geb. Rontgenstr. Nuklearmed. 185, 1149–1166 (2013).
5. Veiseh, O., Gunn, J. W. & Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 62, 284–304 (2010).
6. Lin, B.-L. et al. Superparamagnetic Iron Oxide Nanoparticles-Complexed Cationic Amylose for In Vivo Magnetic Resonance Imaging Tracking of Transplanted Stem Cells in Stroke. Nanomater. Basel Switz.
7, (2017). 7. Guillemot, F. et al. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 6, 2494–2500 (2010).
Figures