3598
Magnetic Particle Imaging as a tool for cell tracking: filling the voids left by MRI1Medical Biophysics, Western University, London, ON, Canada, 2Robarts Research Institute, London, ON, Canada, 3Magnetic Insight Inc, Alameda, CA, United States
Synopsis
Magnetic Particle Imaging (MPI) is emerging as a specific, quantitative and sensitive cellular imaging technique. MPI directly detects a SPIO tracer as positive contrast, and this signal is linearly quantifiable with zero tissue attenuation. Because of this, MPI is well suited for in vivo cell tracking to image tumor associated macrophages (TAMs). TAM content can be used as a biomarker to predict tumor growth and metastatic potential. Mice bearing 4T1 and 168FARN tumors were imaged post intravenous SPIO injection to investigate for the first time, the application of MRI and MPI to differentiate between murine cancer models with varying metastatic potentials.
Background
Magnetic Particle Imaging (MPI) is emerging as a specific, quantitative and sensitive cellular imaging technique. It utilizes iron oxide nanoparticles which are well known and used throughout the MRI community. Unlike MRI, which presents with low specificity and challenging quantification, MPI directly detects the SPIO tracer as positive contrast, and this signal is linearly quantifiable with zero tissue attenuation. These features of MPI make it attractive for quantifying cells in vivo in multiple applications. One well suited application would be imaging tumor associated macrophages (TAMs); they are associated with tumor growth and metastatic potential.1 Increased numbers of TAMs have been correlated with a poor prognosis and they can contribute up to 50% of the mass in breast tumors.2 Previously, cellular MRI has indirectly imaged the spatial distribution of TAMs, through the in situ uptake of iron oxide nanoparticles, visualized as signal voids.3 In this study, we investigated the first application of MRI and MPI to detect and differentiate between murine breast cancer models with varying metastatic potentials.Methods
Female BALB/c mice were implanted with 300,000 4T1 (n=3) or 168FARN (n=3) cells into the inguinal mammary fat pad. These two isogenic murine breast cancer cell lines differ in metastatic potential (high and low, respectively) and consequently, TAM density. Tumour bearing mice were imaged at 3 weeks post cancer cell implantation, 24 hours post intravenous injection of 100μL of VivoTrax (Magnetic Insight Inc.), a superparamagnetic iron oxide (SPIO). One mouse from each group was first imaged on a 3T clinical MRI (General Electric) with a custom-built gradient insert located at Robarts Research Institute. A balanced steady-state free precession pulse sequence was used with an imaging time of ~20 mins. The mice were then sacrificed and fixed in formalin. MPI was performed by Magnetic Insight Inc. with the Momentum MPI scanner (Magnetic Insight Inc.) alongside three reference fiducials of known iron concentration. Iron quantification was performed with VivoQuant (inviCRO) on sagittal projection images with a total MPI scan and reconstruction time of 4 minutes. Tumor tissue was extracted for histological examination.Results
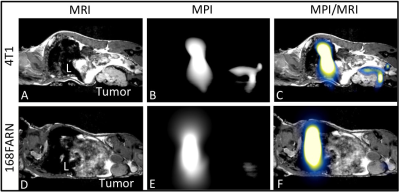
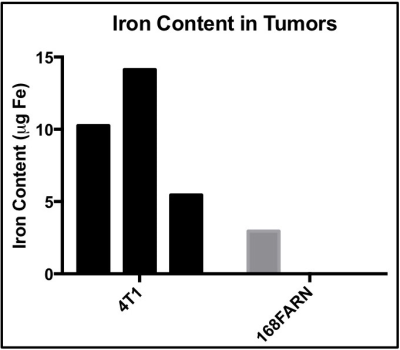
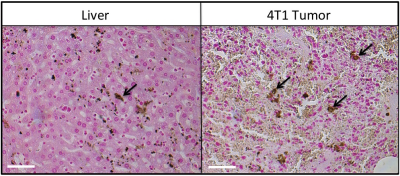
MRI signal voids were observed within the 4T1 tumor (Fig. 1A), predominately in the tumor periphery. Fewer voids with MRI were observed in the 168FARN tumor (Fig 1D). MPI signal was observed in all 4T1 tumors (Fig. 1B) but only in 1/3 of the tumors in the 168FARN group (Fig. 1E), consistent with the lower iron presence observed by MRI. MPI/MRI overlays (Figs. 1C&F) demonstrate a similar location of the MPI positive signal and MRI signal voids, suggesting these are both generated by the SPIO. MPI quantification of iron in the three 4T1 and one 168FARN tumors indicated an uptake of 5.5, 10.3 and 14.1 μg and 2.9 μg of iron, respectively. Signal was always detectable in the liver, due to the inherent phagocytic cells present. Liver and 4T1 tumor sections were stained with nuclear fast red counterstain and suggest iron accumulation in cells, which appear as dark brown (Fig. 3).Conclusions
By combining the high spatial resolution of MRI with the accurate quantification and specificity of MPI, additional information can be obtained on TAM presence and distribution. In addition to aiding therapeutic development, this information could be utilized as a biomarker to predict how aggressive a tumor may be.Acknowledgements
No acknowledgement found.References
1. Obeid E, Nanda R, Fu YX, Olopade OI. The role of tumor-associated macrophages in breast cancer progression. Int J Oncol. 2013;43(1):5-12. doi:10.3892/ijo.2013.1938.
2. Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J Pathol. 2002;196(3):254-265. doi:10.1002/path.1027.
3. Makela AV, Gaudet JM, Foster PJ. Quantifying tumor associated macrophages in breast cancer: a comparison of iron and fluorine-based MRI cell tracking. Sci Rep. 2017;7(42109).
Figures