3594
Imaging glucose-stimulated zinc secretion from the prostate and pancreas using a Mn(II)-based zinc sensor1University of Texas Southwestern Medical Center, Dallas, TX, United States, 2University of Texas at Dallas, Richardson, TX, United States
Synopsis
Imaging glucose-stimulated zinc secretion (GSZS) from secretory tissues has proven useful at assessing organ function and health; current probes to detect zinc secretion by MRI have so far been limited to gadolinium-based sensors. In this work we introduce a manganese-based zinc sensor and show that pancreatic and prostatic zinc detection is not compromised when using Mn instead of Gd for imaging GSZS in vivo. Biodistribution studies indicate that the Mn-based sensor is cleared intact after renal filtration but degraded during hepatobiliary clearance.
Purpose
We have previously reported that Gd-based zinc sensors detect glucose-stimualted zinc secretion (GSZS) from the pancreas during glucose stimulated insulin secretion and from the prostate during secretion of zinc ions from prostatic epithelial cells. Given the current concerns associated with gadolinium deposition and nephrogenic systemic fibrosis after repeated usage of some Gd-based MRI contrast agents, we introduce an effective manganese alternative for the MRI detection of GSZS.Methods
MnL Synthesis: The precursor for the ligand was synthesized following previously published protocols1. Bis(pyridine-2-ylmethyl)ethane-1,2-diamine was coupled to the precursor using PyAOP and DIPEA. The tert-butyl groups were hydrolyzed using TFA to obtain the ligand. MnCl2 at pH 6.5 was used to incorporate Mn2+.
Relaxometry: Varying concentrations of MnL (0 – 1mM) were incubated with 2mM ZnCl2, and 0.6mM human serum albumin (HSA) at 37 °C. T1 and T2 measurements were obtained at 1.5 T using a Bruker relaxometer. T1 was obtained by an inversion recovery sequence (IR, first pulse = 10ms, TR = 10s, TI = 10-2500ms), and T2 by a Carr-Prucell-Meiboom-Gill (CPMG, TE/TR = 5/10000ms, NE = 700).
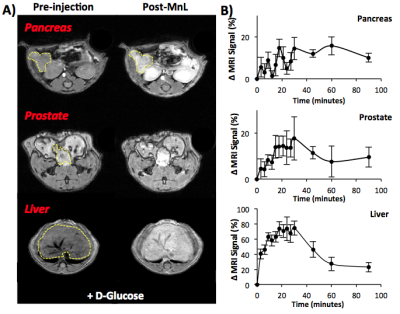
In vivo MRI: Male C57Bl6 mice were imaged at 4.7 T and 9.4 T using Varian/Agilent scanners. Mice were anesthetized with 2-5% isofluorane/oxygen mixture and their body temperature was maintained at 37°C using heated airflow. Two ge3d T1-weighted scans were obtained as a baseline (TE/TR = 1.69/3.35 ms, Average = 4, θ=20°). Mice then received 0.07 mmol/kg MnL i.v. plus 2.2 mmol/kg D-Glucose i.p. and sequential 3d T1-weighted scans were obtained for 90 minutes until clearance of the agent was evident. Images were analyzed using ImageJ, organs of interest were identified and ROIs were measured and normalized to muscle ROIs within the same slice and time point. The change in MR signal intensity is reported as a percentage compared to pre-injection scans.
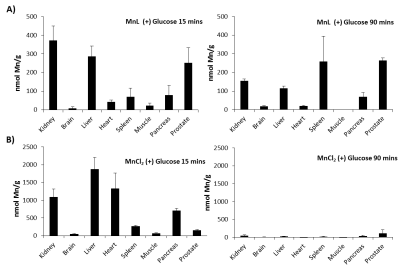
Tissue ICP-AES: Biodistribution of MnL was measured in tissues 15 and 90 min after injection of (1) 0.07 mmol/kg MnL plus 2.2 mmol/kg D-glucose, (2) 0.07 mmol/kg MnL plus saline, and (3) 0.035 mmol/kg MnCl2 plus 2.2 mmol/kg D-glucose into male C57Bl6 mice. The tissue weights were recorded and digested in aqua regia for 24 hours, the digested tissue was heated to 120 °C until evaporated and re-suspended in 0.5 M HCl. For ICP-AES, Mn, Fe, and Zn concentrations were obtained by comparing to a known metal standard.
Results and Discussion
The newly synthesized MnL zinc sensor shows strong zinc binding capabilities (KD(Zn) = 90 nM), relaxometry showed that MnL has a r1/ r2 = 3.5/6.0 mM-1s-1 and that upon binding to Zn2+ and HSA, r1/ r2 = 14.1/22.4 mM-1s-1 (Figure 1). The in vivo images show that MnL is readily detected in T1-weighted images; Figure 2A illustrates enhancement in the pancreas, prostate, kidneys, and liver at 4 minutes post injection. Figure 2B shows the normalized signal change from each organ and washout after 90 minutes. MnL appears to be excreted as an intact complex after renal filtration (as observed by LC-MS of urine), but degraded during hepatobiliary clearance (as observed by LC-MS of bile). Control experiments showed marginal enhancement in the prostate and pancreas when D-glucose was not co-injected suggesting that MnL is uniquely sensitive to zinc. Tissue measurements of Mn content by ICP-AES show the clearance path and biodistribution. Figure 3A illustrates that after 15 minutes post-injection, MnL (presumably the chelated form) is found primarily in the kidneys, liver, pancreas, and prostate. After 90 minutes, the Mn content was greatly diminished in kidney and liver tissues but accumulated in the spleen and in the prostate. The later observation may reflect Mn in the prostatic urethra during excretion of the agent. Figure 3B summarizes the tissue biodistribution of Mn after injection of MnCl2 i.v. and show that MnCl2 is excreted renally and more overwhelmingly via the hepatobiliary system. Accumulation in the heart is indicative of unchelated Mn2+ as observed in the mice injected with MnCl21, while the MnL distribution shows no accumulation in the heart indicating that MnL remains intact during circulation.
Conclusions
In summary, a zinc-responsive Mn-based MRI contrast agent has been used to image GSZS in mice, similar to that reported previously using Gd-based agents2,3. The tissue Mn content showed that the MnL chelate is excreted intact through renal filtration, and appears to be degraded in the liver and excreted in bile as free Mn2+ ions. These data suggest that a Mn-based zinc sensor provides an alternative to the use of Gd agents for detection of GSZS by MRI.Acknowledgements
Financial support from the National Institutes of Health (P41- EB015908, R01-DK095416) and the Robert A. Welch Foundation (AT-584) is gratefully acknowledged.References
1. Gale, E. M., Atanasova, I. P., Blasi, F., Ay, I. & Caravan, P. A Manganese Alternative to Gadolinium for MRI Contrast. J Am Chem Soc 137, 15548-15557, doi:10.1021/jacs.5b10748 (2015).
2. Yu, J. et al. Amplifying the sensitivity of zinc(II) responsive MRI contrast agents by altering water exchange rates. J Am Chem Soc 137, 14173-14179, doi:10.1021/jacs.5b09158 (2015).
3. Esqueda, A. C. et al. A new gadolinium-based MRI zinc sensor. J Am Chem Soc 131, 11387-11391, doi:10.1021/ja901875v (2009).
Figures