3593
MR and optical imaging study to evaluate effects on bioorthogonal click therapy in prostate cancer mouse models1Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Laboratory of Structural Biology, Institute of Biotechnology CAS, Vestec, Czech Republic, 3Department of Oncology, The Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Click therapy is a new therapeutic strategy for cancers minimizing systemic toxicity and enhancing therapeutic efficacy. For this strategy, slow internalization of pretargeted cell surface receptors gives a sufficient window for clearance of the unbound pretargeting component before administration of delivery components. Here, we tested click therapy in PSMA(+) PC3-PIP and PSMA(-) PC3-Flu (control) tumors to evaluate delivery and internalization of components. We observed that internalization of 5D3 antibody is fast, which may interfere with the delivery of therapeutic components. In large tumors with leaky vasculature, 5D3 antibody can effectively extravasate leading to the cluster formation with delivery component in tumor microenvironment.
Introduction
Prostate cancer (PCa) is the second most frequently occurring cancer in men with approximately 900,000 new cases of PCa detected each year.1 Prostate-specific membrane antigen (PSMA) is the clinically validated marker for PCa; it is expressed on the surface of malignant PCa cells and currently is being explored as a target for specific imaging and therapy.2 PSMA is an integral membrane glycoprotein (MW ~ 100 kDa) that contains a short amino-terminal cytoplasmic domain, a single transmembrane domain, and an extracellular protease domain. PSMA is extensively expressed in both primary prostate adenocarcinomas and bone and lymph metastases.3 PSMA is also expressed in the vasculature of multiple non-prostate tumors.4 Bioorthogonal click therapy is a recently developed drug delivery strategy based on the sequential delivery of the pretargeting monoclonal antibody (mAb), followed by the drug-loaded nanocarrier, which will react with each other by bioorthogonal click chemistry.5,6 Pharmacokinetics and cellular internalization of the components play a vital role in this strategy. In this study, we evaluated internalization of the pretargeting mAb in PC3-PIP cells and delivery of therapeutic components in preclinical models of PCa. While rapid internalization of the 5D3 mAb was detected in PCa cells, fast accumulation of components in tumors due to the EPR effect resulted in efficient formation of therapeutic complexes in vivo.Methods
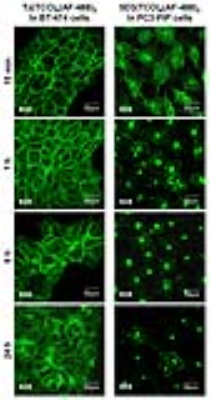
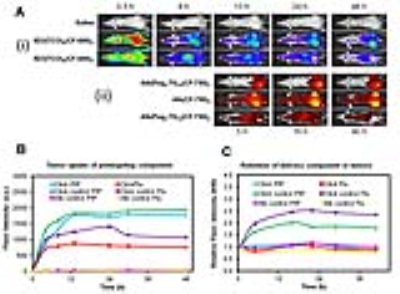
The anti-PSMA 5D3 antibody was conjugated with trans-cyclooctene (TCO), labeled with a suitable fluorophore for in vitro and in vivo optical imaging and used as pretargeting component (Figure 1). Trastuzumab (Tz) mAb was used in control experiments with HER2+ breast cancer cells. The drug delivery component, albumin (Alb), was conjugated with pegylated tetrazine (Peg4-Tt) and labeled with a suitable fluorophores for in vitro and in vivo imaging (Figure 1). In cell studies, PSMA(+) PC3-PIP and HER2(+) BT-474 cells were treated with anti-PSMA 5D3(TCO)6(AF-488)2 and anti-HER2 Tz(TCO)6(AF-488)2 mAbs, respectively (20 µg/mL for 20 min at 37°C), and fixed at 15 min, 1, 6, 24 h. Confocal images were acquired using a Zeiss LSM510-Meta microscope. In vivo optical imaging was performed in athymic nude male mice inoculated with PC3-PIP (PSMA+, left flank) and PC3-Flu (PSMA-, right flank) dual tumors using a Xenogen IVIS imaging system. The pretargeting component, 5D3(TCO)6(CF-680)2, was administered at a dose of 10 mg/kg. The drug delivery component, Alb(Peg4-Tt)10(CF-750)2 or Alb(CF-750)2 as control (100 mg/kg), was administered after 8 h to allow for tumor accumulation and systemic clearance of the pretargeting agent and optical imaging was continued for 48 h. Vascular permeability in the same set of mice were determined by dynamic T1-weighted MRI with gadodiamide (OmniscanTM, 0.2 mmol/kg, i.v.) using a horizontal bore, preclinical 9.4T Bruker Biospec MR scanner.Results
In vitro confocal images of HER2(+) BT-474 and PSMA(+) PC3-PIP cells treated with Tz(TCO)6(AF-488)2 and 5D3(TCO)6(AF-488)2, respectively, taken at different time points, are shown in Figure 2. In vivo optical images of mice treated with 5D3(TCO)6(CF-680)2 followed by Alb(Peg4-Tt)10(CF-750)2 or Alb(CF-750)2 were taken up to 48 h post-injection (Figure 3A). Tumor uptake of the pretargeting component 5D3(TCO)6(CF-680)2 and drug delivery components Alb(Peg4-Tt)10(CF-750)2 or Alb(CF-750)2 are shown in Figure 3 B&C, respectively. Figure 4 shows representative T1-weighted FLASH MR images of click-treated and click-control mice. Subtraction images show the contrast enhancement in the tumors 30 min post-injection of gadodiamide. In vivo MRI study revealed significantly increased extravasation of contrast in peripheral region of large tumors.Discussion
Rapid internalization of the pretargeting mAb diminishes the click reaction between two components on the cell surface. Unlike trastuzumab, anti-PSMA 5D3 antibody was rapidly internalized within 15-30 min in vitro. In vivo optical images demonstrated efficient binding and retention of 5D3 antibody in PC3-PIP tumors and low binding in PC3-Flu tumors. However, pharmacokinetics of the drug delivery component were more aligned with vascular properties of the tumors, presumably due to the EPR effect (Figure 3). This was confirmed by MR images, where low extravasation was observed in small tumors, whereas large tumors had higher contrast uptake. These data suggest that in pretargeting click therapeutic strategy, the pretargeting and delivery components can accumulate both specifically and nonspecifically through active and passive targeting, respectively. While specific formation of the therapeutic complexes on the surface of the target cancer cell is a preferable mechanism of action, pretargeting and delivery components can also undergo click reactions and form therapeutic complexes in the interstitium of tumors, which do not express the target receptor.Conclusion
The internalization of 5D3 antibody is very fast, which may interfere with the delivery of therapeutic components in pretargeting click therapy. In large tumors with leaky vasculature, both components can effectively extravasate and be retained due to the EPR effect leading to the click therapeutic cluster formation in the interstitium of tumors.Acknowledgements
This study was supported by the CDMRP/Department of Defense (W81XWH-16-1-0595) and NIH/National Cancer Institute (R01CA209884).References
1. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN. Int J Cancer 2010;127:2893-2917.
2. Olson WC, Israel RJ. Antibody-drug conjugates targeting prostate-specific membrane antigen. Front Biosci. 2014;19:12-33.
3. Israeli RS, Powell CT, Fair WR, et al. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res 1993;53:227-230.
4. Chang SS, Reuter VE, Heston WD, et al. Metastatic renal cell carcinoma neovasculature expresses prostate-specific membrane antigen. Urology 2001;57:801-805.
5. Hapuarachchige S, Zhu W, Kato Y, et al. Bioorthogonal, two-component delivery systems based on antibody and drug-loaded nanocarriers for enhanced internalization of nanotherapeutics. Biomaterials 2014;35(7):2346-2354.
6. Hapuarachchige S, Kato, Y, Artemov D, Bioorthogonal two-component drug delivery in HER2(+) breast cancer mouse models. Sci Rep 2016;6:24298.
Figures