3592
Detecting prostatic zinc (II) loss in a TRAMP prostate cancer model by glucose-stimulated zinc (II) secretion in vivo with MRI1University of Texas Southwestern Medical Center, Dallas, TX, United States, 2McMaster University, Hamilton, Canada, 3Diamond Light Source, Harwell, United Kingdom, 4University of Texas at Dallas, Richardson, TX, United States
Synopsis
We have previously found that glucose stimulates the secretion of zinc stores from epithelial cells in the prostate gland, and that this glucose-stimulated zinc secretion (GSZS) can be detected in vivo by a Gd-based zinc sensor with T1-weighted MRI. Here we elucidate the mechanisms of enhancement in the healthy prostate and loss of enhancement in the malignant prostate by GSZS MRI and Synchrotron-Radiation X-Ray Fluorescence (SR-XRF). GSZS MRI detects the unique loss of zinc content in the lateral lobe of the malignant prostate as observed by SR-XRF with a Gd-based high affinity Zn2+ sensor.
Purpose
The prostate has the highest zinc content in the body and is dramatically reduced in prostate cancer (PCa)1. We recently reported that the healthy prostate releases Zn2+ ions in response to a glucose challenge and this response is easily detected by T1-weighted MRI in the presence of a zinc sensor. We then showed that glucose-stimulated zinc secretion (GSZS) can be used to monitor PCa progression in a mouse model.2 Here, we evaluated the distribution of Zn2+ within the gland before and after GSZS to fully understand the mechanisms of contrast enhancement and loss in healthy mice and malignant PCa tissues.Methods
GdL1 and GdL2 Synthesis: GdDO3A was linked to a low affinity Zn2+-binding moiety to yield GdL2 or to a high affinity Zn2+-binding moiety to yield GdL1. The synthetic details are reported elsewhere.3
In vivo MRI: Male C57Bl6 mice and Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) mice were anaesthetized with isofluorane and imaged at 9.4T using a Varian/Agilent scanner. Two ge3d T1-weighted scans were obtained (TE/TR=1.69/3.35ms, Average=4, θ=20°) and mice then received: (1) 0.07mmol/kg GdL1 or GdL2 (i.v.) plus 2.2mmol/kg glucose (i.p.) or (2) 0.07mmol/kg GdL1 plus saline as a control. Immediately after injections, sequential 3D T1-weighted scans were collected over 30min.
Synchrotron-Radiation X-Ray Fluorescence (SR-XRF): Male C57Bl6 and TRAMP mice received 0.07mmol/kg GdL2 and either 2.2mmol/kg glucose or saline. After 10mins post-injection, the prostate was resected, frozen in liq.N2-chilled isopentane, stored at -80°C, and sliced into 50μm-thick sections and mounted on XRF-films. The SR-XRF I18 beam line at the Diamond Light Source was used at 8.2 and 11keV to collect atomic images of Zn, P, Cu, and Gd at 50 and 5μm, respectively. The concentration of each element was obtained by measuring a reference material under identical sample conditions and the net peak areas from the sample spectra were translated into elemental maps (ppm).
Transmission electron microscopy (TEM): Mice received an i.p injection of 2.2mmol/kg glucose or saline and after 10 minutes, the prostate was dissected and each prostate lobe were separated. Standard tissue processing protocols were followed and tissue images were acquired with a Tecnai G2 spirit TEM (FEI) equipped with a LaB6 source.
Results and Discussion
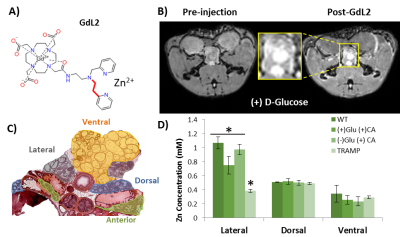
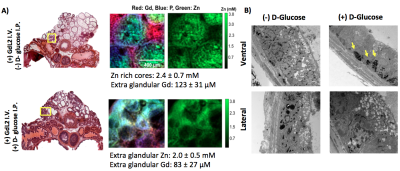
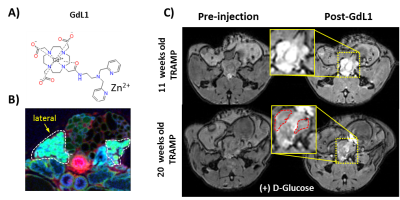
GSZS MRI relies on the binding of a Gd-based agent to zinc and to human serum albumin to generate contrast enhancement. Here we tested the effects of altering the affinity to Zn2+ in the agent on prostate GSZS MRI in vivo. When a low Zn2+ affinity agent, GdL2 (KD(Zn) = 2.35μM) (Fig.1A) was used, the agent showed limited prostatic enhancement showing only slight hyperintensities in the lateral lobe (Fig.1B). Elemental maps of healthy and TRAMP prostates obtained by SR-XRF indicate that the local tissue concentration of Zn2+ is highest in the lateral lobe and that the loss of Zn2+ content in PCa is observed only in the lateral lobe (Fig.1D). High resolution SR-XRF of the lateral lobe revealed zinc-rich acinar gland lumens (2.4 ± 0.7mM) and when stimulated by glucose, Zn2+ was found in the periphery of the glands (2.0 ± 0.5mM) (Fig.2A). TEM of prostates dissected after glucose injection showed that electron dense, presumably zinc-rich granules, migrate from the lumen to the basal lamina of glands (Fig.2B). Given the confirmed secretion of such large concentrations of zinc, we could perhaps improve detection by modifying the affinity to zinc in the probe. Both zinc and citrate are known to be elevated in the healthy prostate4 so it would not be unreasonable to assume that Zn2+ may be weakly associated with citrate (KD = 10μM)5 and that both are co-secreted during GSZS. Should this be true, one would expect to find differences between a low and a high affinity zinc probe due to competition between the probe and citrate. This hypothesis was tested by performing the same experiment with a high affinity zinc probe, GdL(KD(Zn)=118nM) (Fig.3A). In this case, one detects selective MR contrast enhancement of the lateral lobe in healthy TRAMP mice (Fig.3C), consistent with parallel SR-XRF zinc maps (Fig.3B). TRAMP mice were monitored over time and as predicted by SR-XRF, in fully developed PCa, the only significant loss of enhancement was observed in the lateral lobe (Fig.3C).Conclusions
In summary, we demonstrated the glucose-stimulated movement of Zn2+ ions in the mouse prostate by SR-XRF that is consistent with GSZS MRI. Furthermore, we showed that a high affinity zinc sensor, GdL1, shows preferential glucose-stimulated MRI enhancement of the lateral lobe and that there is loss of enhancement (as observed by MRI) and zinc content (as measured by SR-XRF) in fully developed PCa.Acknowledgements
Financial support from the National Institutes of Health (P41- EB015908, R01-DK095416) and the Robert A. Welch Foundation (AT-584) is gratefully acknowledged.References
1 Kelleher, S. L., McCormick, N. H., Velasquez, V. & Lopez, V. Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv Nutr 2, 101-111, doi:10.3945/an.110.000232 (2011).
2 Clavijo Jordan, M. V. et al. Zinc-sensitive MRI contrast agent detects differential release of Zn(II) ions from the healthy vs. malignant mouse prostate. Proc Natl Acad Sci U S A 113, E5464-5471, doi:10.1073/pnas.1609450113 (2016).
3 Martins, A. F. et al. Second generation MRI sensors with lower affinity for zinc ions improve detection of insulin secretion from the mouse pancreas in vivo. J Am Chem Soc Submitted (2017).
4 Costello, L. C. & Franklin, R. B. Zinc is decreased in prostate cancer: an established relationship of prostate cancer! J Biol Inorg Chem 16, 3-8, doi:10.1007/s00775-010-0736-9 (2011).
5 Dubi, N., Gheber, L., Fishman, D., Sekler, I. & Hershfinkel, M. Extracellular zinc and zinc-citrate, acting through a putative zinc-sensing receptor, regulate growth and survival of prostate cancer cells. Carcinogenesis 29, 1692-1700, doi:10.1093/carcin/bgn027 (2008).
Figures