3587
The effect of liposomal encapsulation on the chemical exchange properties of diamagnetic CEST agents.1Brain repair and rehabilitation, University College of London, London, United Kingdom, 2Department of Chemistry, University College of London, London, United Kingdom, 3Brain Repair and Rehabilitation, University College of London, London, United Kingdom
Synopsis
Liposome encapsulation of glucose or 2-deoxy-D-glucose (2-DG) may be exploited to enhance the CEST signal by reducing the overall apparent exchange rate. Here we aim to construct a complete theoretical model to measure the exchange properties of diamagnetic CEST agents. Experimentally measured exchange rates of glucose and 2-DG in the liposomal system were found to be reduced by one or two orders of magnitude due to the intermembrane exchange between the intra- and extra-liposomal compartment because of restrictions in water transfer imposed by the lipid membrane. These new theoretical and experimental findings are expected to benefit applications of diamagnetic liposomes to image biological processes.
Introduction
We hypothesized that the current challenges of sensitivity and toxicity opposing clinical translation of gluco- and 2-DG-CEST could be overcome by encapsulating high concentrations of the monosaccharides in the aqueous interior of liposomes (1),(2). A better understanding of CEST signal characteristics of these new compounds enables the construction of a full three-site exchange model for quantifying the exchange rate between monosaccharides and intra-liposomal water as a function of the intra-liposomal and extra-liposomal water magnetization. This information can be used in turn to characterise the liposomal system encapsulating monosaccharides and to optimize the experimental parameters to achieve superior saturation efficiency (3). Finally, these liposomal systems might be employed as a novel way to estimate the exchange rate of water across lipid bilayers.Methods
In a first step, a simple two-site exchange between water and monosaccharides can be established. By measuring the exchange rates separately for free and encapsulated monosaccharides in liposomes we can evaluate the effect of the intermembrane exchange on the total exchange rate between monosaccharides and water at different conditions. The steady state magnetization Mwz is given by (4):
$$ Mwz/M0= R1A/(R1p+ R1A(1-DC)) Equation 1
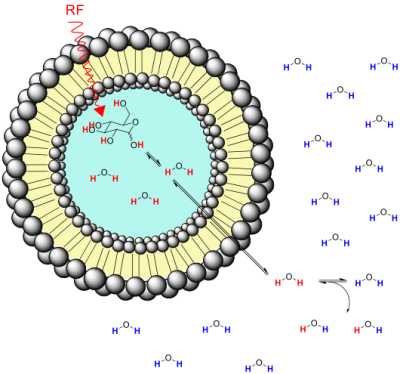
However, the complete system can only be described through a full three-site exchange model (see Figure 1). The hydroxyl protons in monosaccharides are in chemical exchange with the intra-liposomal water, which are in a two-site exchange. Then, the intra-liposomal water, having experienced exchange with OH protons from glucose or 2-DG enters the extra-liposomal space and thereby physically transfers saturated protons to bulk water via the lipid membrane with a rate known as the intermembrane exchange rate. The extra-liposomal water magnetization in steady state conditions can be written as a function of the intra-liposomal water magnetization as follows:
Mwzextra= (1/(R1extra+Rintermebrane .M0intra).(Rintermembrane.M0extra.Mwzintra+ R1extra.M0extra)) Equation 2
where Rintermembrane. M0intra= kextra/intra, and Rintermembrane .M0extra= kintra/extra
The measured signal from the liposomal sample is the sum of the water magnetizations in the intra-liposomal and extra-liposomal space weighted by their equilibrium magnetizations M0intra and M0exrta
Z-spectra were acquired on a 9.4T Agilent MRI scanner using a single-shot single-slice spin-echo (SE) echo planar imaging (EPI) sequence (TR=65.3ms, TE=4.07ms, FOV=20x20mm², slice thickness=5mm, matrix size=64x64) with a saturation train prior to the readout consisting of 151 Gaussian pulses (pulse length = 50ms, 99% duty cycle) at 10 different amplitudes (0.39μT, 0.76μT, 1.17μT, 1.57μT, 1.96μT, 2.35μT, 2.74μT, 3.13μT, 3.52μT, and 4.31μT). Raw data were fitted using either Equation 1 or 2 using the optimization function lsqcurvefit in MATLAB.
Liposome samples: 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) liposomes containing 0.25M glucose or 0.25M 2-DG were formulated in 20% phosphate buffer saline (PBS) at pH 6 and 7 via sonication, extruded to achieve an average diameter of ~200nm and dialysed into 0.25M NaCl solution. The overall sugar concentrations for both liposome samples were enzymatically measured and adjusted to 23mM. Free sugar controls were made up at 23mM in 20% PBS at pH 6 and 7.
Results
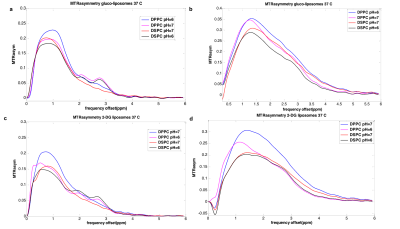
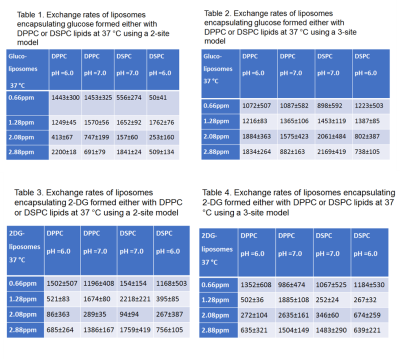
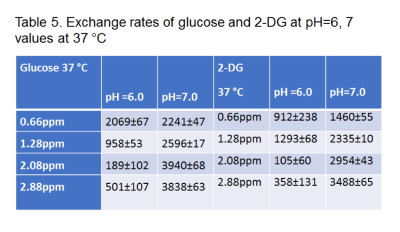
According to our results, the exchange rates calculated either by using a three-site or a two-site exchange model are significantly smaller (p<0.05) than the ones calculated for free monosaccharides using a two-site model. Tables 1-4 display the exchange rates of all the hydroxyl groups of glucose or 2-DG resonating at 0.66ppm, 1.28ppm, 2.08 ppm and 2.88ppm. The exchange rates for free glucose and free 2-DG are also shown for comparison in Table 5. Figure 2 displays the MTR asymmetries of monosaccharides encapsulating glucose or 2-DG at 37 C.Discussion
In this study, we used a rich multipower dataset for calculating the exchange rates of monosaccharide hydroxyl pools inside liposomes. To be able to separate the effects of chemical exchange from physical water transfer across the bilayer barrier we constructed a three-site exchange model which describes such systems in a much more precise way than previously reported. Thus, improved estimates of the exchange rates of hydroxyl protons in liposomes are provided, which were found to be reduced by one or two orders of magnitude compared to chemical exchange rates of free monosaccharide solutions. Our findings and the theoretical development for quantifying the exchange rates of monosaccharides encapsulated in liposomes will be beneficial for future GlucoCEST studies while the same principles can be applied for other diaCEST molecules. Moreover, the potential benefits of this approach include a more detailed understanding of the effects of membrane lipid composition on permeability in both model liposomes used to transport drugs or contrast agents, and in membrane systems designed to mimic the composition of biological cell membranes. This will in turn have implications for the design of new liposomal drug carriers, as well as a better understanding of drug toxicity, pharmacokinetics, and the factors governing membrane fluidity.Acknowledgements
No acknowledgement found.References
Walker-Samuel S, Ramasawmy R, Torrealdea F, Rega M, Rajkumar V, Johnson SP, et al. In vivo imaging of glucose uptake and metabolism in tumors. Nat Med. 2013 Aug;19(8):1067–72. 2.
Nasrallah FA, Pagès G, Kuchel PW, Golay X, Chuang K-H. Imaging brain deoxyglucose uptake and metabolism by glucoCEST MRI. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2013 Aug;33(8):1270–8. 3.
Chan KWY, Bulte JWM, McMahon MT. Diamagnetic chemical exchange saturation transfer (diaCEST) liposomes: physicochemical properties and imaging applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014 Feb;6(1):111–24. 4.
Meissner J-E, Goerke S, Rerich E, Klika KD, Radbruch A, Ladd ME, et al. Quantitative pulsed CEST-MRI using Ω-plots. NMR Biomed [Internet]. 2015 Oct;28(10):1196–1208.
Figures