3586
Labelling of collagen type I templates with a novel naturally derived contrast agent for magnetic resonance imaging in soft tissue engineering1Urology, Radboud University Medical Center; Radboud Institute for Molecular Life Sciences, Nijmegen, Netherlands, 2Experimental Molecular Imaging, Uniklinik RWTH and Helmholtz Institute for Biomedical Engineering, RWTH Aachen University, Aachen, Germany, 3Department of Radiology and Nuclear Medicine, Radboud University Medical Center, Nijmegen, Netherlands, 4Nano4Imaging, Aachen, Germany, 5Urology, Radboud University Medical Center; Radboud Institute for Molecular Life Sciences; Radboudumc Amalia Children's Hospital, Nijmegen, Netherlands
Synopsis
Contrast agents (CA) need to be applied for monitoring tissue-engineered implants by MR imaging. However, currently used CAs have limitations (i.e. negative contrast, label instability). In this study, Hemin-Lysine complex (HL) -as a naturally derived alternative CA- is used for active labeling of hybrid collagen-based templates. HL-labeled templates are clearly identified because of their bright signal in T1-weighted MR images. The signal of labeled templates and thus their integrity could be followed over time when subcutaneously implanted in a mouse model. Thus, loading collagen-based templates with HL appears to be a promising strategy to localize and monitor its fate upon implantation.
Introduction
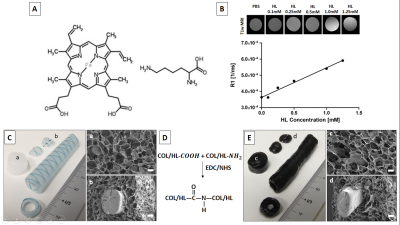
Different porous collagen type I templates have been used for various applications in the field of Tissue Engineering and Regenerative Medicine (TERM) 1–4. However, collagen templates give poor contrast to surrounding tissues in MR images, resulting in problematic localization and consequent non-destructive monitoring of its resorption after transplantation in vivo. This obstacle may be solved by incorporating contrast agents (CAs) such as ultra-small superparamagnetic iron oxide nanoparticles (USPIO) to enhance MRI visibility. However, images may be misinterpreted due to negative contrast by USPIOs. In addition, ´blooming´ effects may occur, and particles may be washed-out independent of collagen remodeling 5,6. Thus, it is essential to develop alternative CAs for collagen type I templates. Hemin is a natural, Fe3+-containing red blood pigment (Figure 1A), and a potentially suitable MRI contrast agent due to the configuration of 3rd orbital electrons. These magnetic properties of hemin make it a promising candidate for enhancing T1-weighted MRI contrast of transplants. Thus, in this study, we explored a hemin-lysine complex (HL) to label collagen type I templates.Materials and Methods
Template preparation
Various collagen type I templates were produced as previously described 1,7 and the hemin-lysine complex (HL) was actively coupled after incubation to collagen type I during crosslinking via N-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) / N-hydroxysuccinimide (NHS) (Figure 1D). Subsequently, HL-loaded hybrid collagen templates analyzed by MR imaging and transplanted subcutaneously in a mouse model and followed up by MRI.
MR Experiments
MRI experiments were performed on a 11.7 Tesla preclinical MR system (BioSpec, Bruker, Germany) equipped with a 1H volume coil with inner diameter of 40 mm.
In vitro MRI: A rapid acquisition with relaxation enhancement (RARE) sequence was employed for acquiring T1 weighted MR images, applying the following parameters: echo time (TE) = 6ms, repetition time (TR) = 600ms, excitation/refocusing pulse 90°/180°, number of slices 20, thickness 0.5 mm, RARE factor 4, field of view (FOV) = 50×50 mm matrix = 256×256 and 8 averages. The scan time was 3mins 5secs. The T1 relaxation properties of hemin incorporated collagen template were estimated with a multi-TR and multi-TE RARE sequence. MR images were obtained at 8 TRs (i.e., 200, 500, 1000, 2000, 3000, 5000, 7000, 10000 ms) and T1 values were estimated by fitting the data to the equation: Mz(TR) = M0 * (1-exp(-TR/T1)). The scan time was 7 mins 39 seconds. Image contrast between the applied scaffold and the background were assessed using the contrast to noise ratio (CNR), which was defined as the difference in signal between the contrast giving tissues divided by the standard deviation of the background signal.
In vivo MRI: High resolution T1 weighted images were acquired with a RARE sequence to visualize the implanted templates in mice at weeks 2, 4 and 12. The corresponding scanning parameters were: TE = 10ms, TR = 560ms, RARE factor 2, number of slices 20, thickness 0.5 mm, FOV = 50×50 mm, matrix = 512×512 and 16 averages. Scan time was 28 mins 40 secs.
Results
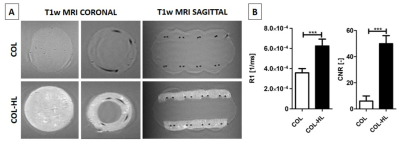
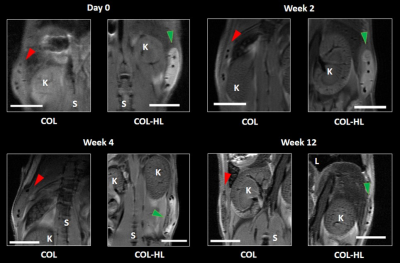
The relaxation rate R1 of hemin-lysine (HL) dissolved in phosphate buffered saline (PBS) was estimated to be (1.8 ± 0.1) x 10-4 / ms (Figure 1B; R² = 0.9850). T1-weighted MR images of HL-labeled collagen type I templates (COL-HL) resulted in increased visualization of collagen when compared with non-labeled templates (COL) (Figure 2A). Analogously, R1 relaxation and contrast to noise ratio (CNR) of this labeled hybrid template was significantly higher when compared to unlabeled hybrid templates (p < 0.0001). R1 relaxation was (6.2 ± 0.7) x 10-4 / ms for HL-COL and (3.6 ± 0.4) x 10-4 / ms for COL alone. CNR was 49.9 ± 6.1 for COL-HL 6.0 ± 3.9 and for COL alone (Figure 2B). Immediately after transplantation, the hybrid COL-HL patch showed clear contrast and could easily be identified (Figure 3) . The collagen component could be distinguished (bright contrast) from the polymeric suture monofilament structure (negative contrast).Discussion/Conclusion
The bio-based hemin-lysine complex (HL) is a promising MRI contrast agent. Hybrid collagen type I templates actively loaded with HL showed enhanced positive contrast in T1-weighted MR images, hence template integrity can be clearly localized and followed longitudinally. Here we show that HL loaded tissue-engineered templates in mice could be clearly distinguished from surrounding tissues on MR images and monitored over time.Acknowledgements
The research leading to these results has received funding from the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme FP7/2007-2013/ under REA grant agreement No. 607868 (iTERM) and applied to European Patent Office (EP16189057).References
(1) Sun, W.; Tiemessen, D. M.; Sloff, M.; Lammers, R. J.; de Mulder, E. L.; Hilborn, J.; Gupta, B.; Feitz, W. F.; Daamen, W. F.; van Kuppevelt, T. H.; Geutjes, P. J.; Oosterwijk, E. Improving the Cell Distribution in Collagen-Coated Poly-Caprolactone Knittings. Tissue Eng Part C Methods 2012, 18 (10), 731–739.
(2) Brouwer, K. M.; Daamen, W. F.; Hoogenkamp, H. R.; Geutjes, P. J.; de Blaauw, I.; Janssen-Kessels, W.; de Boode, W.; Versteeg, E.; Wijnen, R. M.; Feitz, W. F.; Wijnen, M.; van Kuppevelt, T. H. Collagen-Vicryl Scaffolds for Reconstruction of the Diaphragm in a Large Animal Model. J. Biomed. Mater. Res. B. Appl. Biomater. 2014, 102 (4), 756–763.
(3) Sloff,
M.; Simaioforidis, V.; Geutjes, P. J.; Hoogenkamp, H. R.; van Kuppevelt, T. H.;
Daamen, W. F.; Oosterwijk, E.; Feitz, W. F. Novel Tubular Constructs for
Urinary Diversion: A Biocompatibility Study in Pigs. J. Tissue Eng. Regen.
Med. 2016.
(4) Roelofs, L. A. J.; Oosterwijk, E.; Kortmann, B. B. M.; Daamen, W. F.; Tiemessen, D. M.; Brouwer, K. M.; Eggink, A. J.; Crevels, A. J.; Wijnen, R. M. H.; van Kuppevelt, T. H.; Geutjes, P. J.; Feitz, W. F. J. Bladder Regeneration Using a Smart Acellular Collagen Scaffold with Growth Factors VEGF, FGF2 and HB-EGF. Tissue Eng. Part A 2016, 22 (1–2), 83–92.
(5) Mertens, M. E.; Hermann, A.; Bühren, A.; Olde-Damink, L.; Möckel, D.; Gremse, F.; Ehling, J.; Kiessling, F.; Lammers, T. Iron Oxide-Labeled Collagen Scaffolds for Non-Invasive MR Imaging in Tissue Engineering. Adv. Funct. Mater. 2014, 24 (6), 754–762.
(6) Sun, Y.; Geutjes, P.; Oosterwijk, E.; Heerschap, A. In Vivo Magnetic Resonance Imaging of Type I Collagen Scaffold in Rat: Improving Visualization of Bladder and Subcutaneous Implants. Tissue Eng. Part C. Methods 2014, 20 (12), 964–971.
(7) Janke, H. P.; Bohlin, J.; Lomme, R. M. L. M.; Mihaila, S. M.; Hilborn, J.; Feitz, W. F. J.; Oosterwijk, E. Bioinspired Coupled Helical Coils for Soft Tissue Engineering of Tubular Structures – Improved Mechanical Behavior of Tubular Collagen Type I Templates. Acta Biomater. 2017, 59, 234–242.
Figures