3585
Sensing intracellular calcium ions using a manganese-based MRI contrast agent1Bioengineering, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Inspired by the classical work of R. Tsien on cell-permeable calcium specific chelators using readily cleavable acetomethoxy esters (AM) of BAPTA and taking advantage of our own, novel, cell permeable manganese-based contrast agent, we developed a new MRI contrast that displays membrane permeability, cellular accumulation via cleavable ester groups, and physiological sensitivity to calcium. With this breakthrough innovation, we present a series of firsts for functional molecular imaging, reporting chemically and optogenetically induced calcium transients with MRI in living cells.
Introduction
Calcium ions are
the main second messenger of intracellular signaling in many cell types, are commonly considered a direct correlate of neuronal activity. The
development of probes and assays for noninvasive intracellular
calcium signaling is one of the greatest challenges in chemical
neuroscience1,2. Here we present a new manganese-based MRI
contrast agent responsive to calcium fluctuations (ManICS1) and its cell
trappable variant (ManICS1-AM). After cytosolic accumulation and
enzymatic hydrolysis of the labile acetomethoxy esters, ManICS1 can report MRI signal changes in response to
stimuli that elevate intracellular calcium, suitable for in vivo neuroimaging of cellular activity. Calcium-dependent fMRI
will be a breakthrough technique for analysis of neural circuits in animals,
with potential longer term applications in humans.Results and Discussion
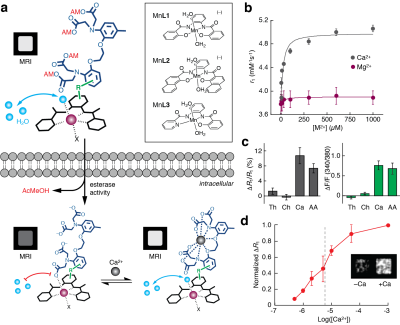
The ManICS sensor consists of a cell permeable contrast agent3 (a) (black complex), a BAPTA-based calcium chelator4 (dark blue), and a linker connecting them (green). Prior to cell entry (top) BAPTA carboxylates are protected with AM esters (red), allowing water exchange (cyan spheres) at the metal center (purple) and T1-weighted MRI contrast (labeled MRI). Once in cells (bottom left), AM esters are cleaved, and sensor enters the calcium-free “off” state (exchange blocked), with low MRI contrast. When calcium binds (bottom right), q and water exchange dynamics increase paramagnetic center, activating the sensor.
ManICS1 shows significant relaxivity (r1) changes over physiologicaly relevant levels of calcium (b) HEK293 cells were labeled with ManICS1-AM (left) or Fura-2-AM (right) and treated with the cell stimulants thapsigargin (Th), carbachol (Ch), calcium ionophore (Ca), or arachidonic acid (AA) (c). Only agents that elicited calcium transients as measured in a Fura2 assay, Ca and AA resulted in significant changes in R1. In a calcium titration in ManICS1-AM-loaded cells permeablized with a calcium ionophore, calcium-induced changes over physiologically relevant concentrations of calcium with a midpoint at [Ca2+] = 5 µM.
Acknowledgements
NIH grants R21-MH102470 and U01-NS090451References
1. Bartelle, B. B., Barandov, A. & Jasanoff, A. Molecular fMRI. J. Neurosci. 36, 4139–4148 (2016).
2. Jorgenson, L. A. et al. The BRAIN Initiative: developing technology to catalyse neuroscience discovery. Philos. Trans. R. Soc. B Biol. Sci. 370, (2015).
3. Barandov, A. et al. Membrane-permeable Mn(III) complexes for molecular magnetic resonance imaging of intracellular targets. J. Am. Chem. Soc. 138, 5483–5486 (2016).
4. Grynkiewicz, G., Poenie, M. & Tsien, R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 (1985).
Figures