3584
Calcium-dependent molecular fMRI using a magnetic nanosensor1Bioengineering, MIT, Cambridge, MA, United States, 2Brain & Cognitive Sciences, MIT, Cambridge, MA, United States
Synopsis
Superparamagnetic iron oxide nanoparticles are a modular platform technology for sensors with sub nM sensitivity and robust biomedical applications. In this work we engineer nanoparticle sensors that display Ca2+ dependent aggregation and demonstrate the first functional MRI study of Ca2+ dynamics as well as the first in vivo demonstration of a dynamic nanosensor.
Introduction
Calcium ions are ubiquitous signaling molecules in all multicellular organisms, where they mediate diverse aspects of intracellular and extracellular communication over widely varying temporal and spatial scales1. Although techniques for mapping calcium-related activity at high resolution by optical means are well established, there is currently no reliable method to measure calcium dynamics over large volumes in intact tissue. Here we address this need by introducing a family of magnetic calcium-responsive nanoparticles (MaCaReNas) that can be detected by magnetic resonance imaging (MRI). MaCaReNas respond within seconds to [Ca2+] changes in the 0.1-1.0 mM range, suitable for monitoring extracellular calcium signaling processes in the brain. We show that the probes permit repeated detection of brain activation in response to diverse stimuli in vivo. MaCaReNas thus provide a tool for calcium activity mapping in deep tissue and offer a precedent for development of further nanoparticle-based sensors for dynamic molecular imaging with MRI.Results and Discussion
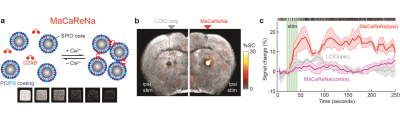
The design of MaCaReNas consists of lipid coated super paramagnetic iron oxide nanoparticles SPIOs and the Ca2+ dependent lipid binding C2AB Ca2+ (a). Increasing levels of Ca2+ cause aggregation of nanoparticles increasing R2 and leading to darker T2 contrast2. Striatal extracellular Ca2+ response to stimulation of the Medial Forebrain Bundle (b). Dopamine release causes an increase in neuronal activity and a corresponding decrease in extracellular Ca2+ that outlasts the duration of stimulous3,4. Brain stimulation leads to an initial response followed by Ca2+ depression. Single unit electrophysiology, shows a increase in neuronal firing well correlated with an increase signal from MaCaReNa (c).
These results
constitute a first for molecular fMRI by reporting Ca2+ in response to brain stimulus. These data demonstrate how extracelluar Ca2+ correlates with neuronal activation, providing new insight into the molecular basis of brain activity5. MaCaReNas also offer applications in translational studies of epilepsy and Alzheimer’s disease, which both have characterized pathologies of extracelluar Ca2+ regulation6. Finally, this work presents an exciting new direction for super paramagnetic iron oxide nanoparticles, demonstrating how this technology can offer a modular platform for in vivo sensing with MRI. Future work will include extending this approach to other target molecules as well as broader delivery and faster kinetics of MaCaReNas
Acknowledgements
Project funding was provided by NIH grants R01-DA038642, DP2-OD2114, BRAIN Initiative award U01-NS090451, and an MIT Simons Center for the Social Brain Seed Grant to AJ. SO was supported by a JSPS Postdoctoral Fellowship for Research Abroad and by an Uehara Memorial Foundation postdoctoral fellowship. ER was supported by a Beatriu Pinos Fellowship from the Government of Catalonia.References
1. Berridge, M. J., Lipp, P. & Bootman, M. D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 (2000).
2. Atanasijevic, T., Shusteff, M., Fam, P. & Jasanoff, A. Calcium-sensitive MRI contrast agents based on superparamagnetic iron oxide nanoparticles and calmodulin. Proc. Natl. Acad. Sci. U.S.A. 103, 14707–14712 (2006).
3. Hernandez, G. et al. Prolonged rewarding stimulation of the rat medial forebrain bundle: neurochemical and behavioral consequences. Behav. Neurosci. 120, 888–904 (2006).
4. Lee, T., Cai, L. X., Lelyveld, V. S., Hai, A. & Jasanoff, A. Molecular-Level Functional Magnetic Resonance Imaging of Dopaminergic Signaling. Science 344, 533–535 (2014).
Figures