3583
Using single 19F-probe for multiplexed imaging with 19F-CEST MRI1Organic chemistry, Weizmann Institute of science, Rehovot, Israel, 2chemical services, Weizmann Institute of science, Rehovot, Israel, 3Chemistry, Tulane University, New Orleans, LA, United States
Synopsis
Heteronuclear-CEST imaging presents several unique properties when compared to 1H-CEST, which is based on water, including background-free signals, quantifyability and ability to monitor low concentrations of targets. Here we present the performance of the CEST approach in 19F-MRI framework for mapping multiple targets in a “multicolor” fashion. Specifically, the difference in binding kinetics between a 19F-agent and a molecular target (i.e., macrocyclic molecular host) and the different Δw values obtained in 19F-NMR lead to a clear 19F-CEST characteristics. The large 19F-CEST effect obtained and its Δw dependency allow the mapping of two molecular targets simultaneously using single 19F-probe.
Introduction
The complexity of biology attracts scientists and clinicians from a wide range of fields. Indeed, optical based dyes have been widely developed and used to study such complexity in a multicolor imaging fashion. However, their light signal source calls for alternatives that could be performed on live intact subjects. In MRI, both diaCEST1 and paraCEST2 based probes have been designed and used for multiplexed imaging mimicking optical imaging capabilities exploiting the Δw of the exchangeable proton in the designed agents. Here, we show that performing the CEST approach in 19F-MRI framework allows obtaining “multicolor” imaging features of multiple targets using single 19F-agent. Based on different binding kinetics in host:guest molecular systems and distinct Δws obtained in 19F-MR, a novel platform for multiplexed MR imaging is presented.Methods
All NMR and MRI experiments were performed on 9.4T scanners (Bruker, Germany). 19F-CEST NMR data were acquired as follows: A pre-saturation pulse with a length (tsat) of 3 sec was applied prior to the 900 RF pulse. The saturation pulse strength was set to 150Hz for all samples. The frequency of the pre-saturation pulse was swept from Δω=+6.1ppm to Δω=-6.1ppm in 100Hz=0.26ppm steps relative to the resonance of the free 19F-agent (i.e., fluoroxene, set to 0ppm for convenience). For each frequency offset (MΔωi), the following parameters were used: NS=8, TR=15. For 1H-MRI, a FLASH sequence was used with the following parameters TR/TE=100/6 ms; 2 mm slice; FOV=2.56×2.56 cm2; matrix size=128×128. For 19F-MRI, RARE sequence was used TR/TE=6000/3.64 ms; 10 mm slice thickness; FOV=3.2×3.2 cm2; matrix size=64×64. For the 19F-CEST MRI the frequency of the pre-saturation pulse (B1=3.6 μT/ 3000 ms) was swept from Δω=+5ppm to Δω=-5ppm in 100Hz=0.26ppm steps.Results and discussion
Recently, the use of the CEST approach in 19F-NMR framework for studying the binding kinetics in several host:guest supramolecular systems was shown3-4. In this approach, the magnetization of a low concentration of host:guest complex is transferred to the signal of the free guest through a dynamic exchange process. In the present study, two supramolecular systems composed of molecular hosts (either cucurbit[n]uril, CB[7]; or octa acid, OA, Fig. 1a) and 19F-guest (fluoroxene, Fig. 1a right panel) were used. Following calculation of the relaxation parameters of the fluorinated agent fluoroxene (T1=4.34 sec and T2=3.33 sec) the 19F-CEST characteristics of CB[7]:fluoroxene and OA:fluoroxene systems in PBS were studied and summarized in Fig. 1b. Obviously, without a molecular host (CB[7] or OA) in the sample, no 19F-CEST effect was observed (Fig. 1b, red curve). Interestingly, when low concentration (50 μM) of molecular host (CB[7] or OA) was added into the studied solution a large CEST effect was obtained (45-50% signal change for 1:100 host:guest ratio) for both studied systems. Importantly, while for CB[7]:fluoroxene system the obtained 19F-CEST effect was at Δw of +1.6 ppm (Fig. 1b, purple curve), the effect for OA:fluoroxene system was opposite, at Δw of -2.0 ppm (Fig. 1b, green curve). This observation, of Δw-CEST dependence, encouraged us to use this platform for multiplexed 19F-MRI. A phantom consisting of 4 different host:guest pairs (1:100 molar ratio, 50 μM of host and 5 mM 19F-guest) in PBS were prepared as shown in Fig. 2a) – CB[6]:fluoroxene, CB[7]:fluoroxene, β-cyclodextrin:fluoroxene and OA:fluoroxene. As expected, 1H-MRI and 19F-MRI did not reveal any difference between the samples in the phantom. However, by performing the 19F-CEST MRI experiment a multicolor imaging characteristic was obtained. While the pre-saturation pulse was applied at Δw=+1.6 ppm, the CEST effect was obtained only from the CB[7] (50 μM) containing tube. Contrary, while the pre-saturation pulse was applied at Δw=-2.0 ppm, the CEST effect was obtained only from the OA (50 μM) containing tube. These observations are shown as pseudo-multicolor imaging map overlaid on 1H-MR image demonstrating the capabilities of using the proposed platform for multiplexed MR imaging. It is important to mention that the low concentrations of molecular targets used here (50 μM of CB[7] or OA) can not be directly detected with 19F-MRI. However, the use of 19F-CEST allows the observation of these targets (50 μM) with the sensitivity of the free 19F-agent (5 mM).Conclusion
Conclusion
Conclusion
We have demonstrated the feasibility of applying the 19F-CEST methodology on a variety of host:guest systems to amplify 19F-MR signals of low concentration targets (~mM levels of molecular host). Capitalizing on the different Δw obtained for the 19F-guest upon encapsulation, a multicolor 19F-CEST MRI can be obtained. By targeting the molecular hosts (CB[7] or OA) to multiple low-concentration targets the proposed platform has the potentiality to monitor such targets, simultaneously, using single 19F-probe.Acknowledgements
No acknowledgement found.References
1. Liu, G.; Moake, M.; Har-el, Y.-e.; Long, C. M.; Chan, K. W. Y.; Cardona, A.; Jamil, M.; Walczak, P.; Gilad, A. A.; Sgouros, G.; van Zijl, P. C. M.; Bulte, J. W. M.; McMahon, M. T., In vivo multicolor molecular MR imaging using diamagnetic chemical exchange saturation transfer liposomes. Magnetic Resonance in Medicine 2012, 67 (4), 1106-1113.
2. Ferrauto, G.; Castelli, D. D.; Terreno, E.; Aime, S., In vivo MRI visualization of different cell populations labeled with PARACEST agents. Magnetic Resonance in Medicine 2013, 69 (6), 1703-1711.
3. Avram, L.; Iron, M. A.; Bar-Shir, A., Amplifying undetectable NMR signals to study host-guest interactions and exchange. Chemical Science 2016, 7, 6905-6909.
4. Avram, L.; Wishard, A. D.; Gibb, B. C.; Bar-Shir, A., Quantifying Guest Exchange in Supramolecular Systems. Angewandte Chemie International Edition 2017, In Press
Figures
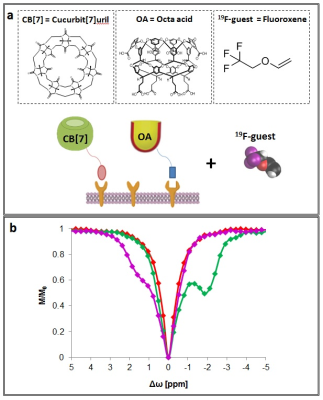
(a) Chemical structure of cucurbit[7]uril (CB[7]), octa acid (OA), and Fluoroxene, followed by a schematic description of the multicolored host-guest probes system.
(b) 19F-CEST-NMR characterization of the CB[7]:fluoroxene (1:100 ratio) and OA:fluoroxene (1:100 ratio) systems.
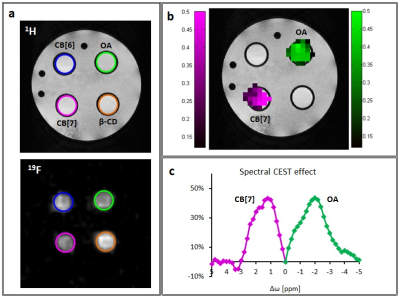
(a) 1H-MRI and 19F-MRI of a 4-sample tube containing CB[6]:fluoroxene (blue circle) CB[7]:fluoroxene (magenta circle), β-cyclodextrin:fluoroxene (orange circle) and OA:fluoroxene (green circle) host-guest systems.
(b) 19F-CEST map overlaid on 1H-MRI obtained for all samples exhibiting significant effect for the CB[7]:fluoroxene (Δw=+1.6 ppm) and OA:fluoroxene (Δw=-2.0 ppm) systems.
(c) The MTRasym plots obtained from the map shown in (b).