3582
An Elastase Activity Reporter for EPR and OMRI as a Line-Shifting Nitroxide.1UMR CNRS 5536, bordeaux, France, 2UMR CNRS 7273, marseille, France, 3EA7426, Faculté de Médecine Lyon Sud, Pierre Bénite, France
Synopsis
Neutrophils secrete proteases at inflammation sites leading to protease/inhibitor imbalance. Among them, neutrophil elastase (NE) is responsible for lung degradation via elastin fragmentation. Monitoring protease/inhibitor status non-invasively would be an important diagnostic tool. We present Meo-Suc-(Ala)2-Pro-Val-nitroxide, a line-shifting elastase activity probe for Electronic Paramagnetic Resonance (EPR) spectroscopy and Overhauser-enhanced Magnetic Resonance Imaging (OMRI). Fast and sensitive with Km= 15 ± 2.9 µM, kcat/Km= 930000 s-1.M-1, this substrate was assessed with bronchoalveolar lavage samples from Pseudomonas pneumonia mouse model. We observed a clear difference between wild type and NE deficient animals. These results can lead to new in vivo diagnostic methods and lung protection.
Introduction
Material & Methods
The OMRI system used in all experiments is an EPR cavity (Bruker, Wissemburg, France) inserted at the center of Cirrus Open 0.2 T MRI system (MRI Tech, Canada). This permanent magnet at 0.193 T is operated at a proton frequency of 8.24 MHz and maximal field gradient strength was 20 mT/m in the three directions of space. EPR acquisitions were performed at 25°C with an EMXnano EPR spectrometer (BRUKER, Germany). Initial velocities of the kinetics of substrate to product formation were analysis. Substrate and product concentrations were obtained using Spincount calibrated module.Results
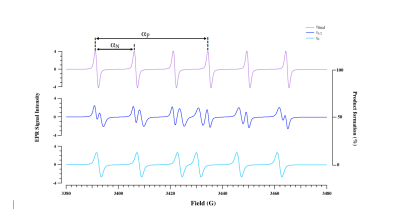
Main point is that hydrolysis of Meo-Suc-(Ala)2-Pro-Val-nitroxide enol ester into the ketone form by neutrophil elastase (NE) showed, in EPR, a difference of 4.9 G in phosphorus hyperfine coupling constants before and after hydrolysis. Substrate and product spectra are sufficiently resolved to avoid peaks overlapping, allowing individual quantification (Figure 1).
Michaelis-Menten results suggest that this substrate is fast and sensitive with Km = 15 ± 2.9 µM, kcat/Km = 930000 s-1.M-1 and Km = 25 ± 5.4 µM, kcat/Km = 640000 s-1.M-1 (R and S isomers, respectively)4. Both isomers display similar constants, having Km 4-7 fold higher than the reference chromogenic substrate Meo-Suc-(Ala)2-Pro-Val-pNA and a catalytic constant 6–9 fold higher5 suggesting a better interaction with the P’ part.
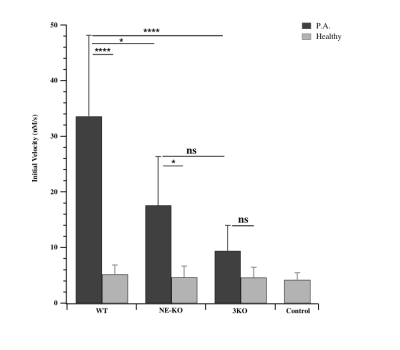
NE activity was assessed with bronchoalveolar lavage samples from Pseudomonas pneumonia mouse model characterized by an acute pulmonary inflammation6 (Figure 2). Infected WT mice BALs contained maximal activity. Interestingly, activity in infected NE-KO dropped by 50%. Clearly, substrate hydrolysis is due to active NE which represents a selective marker of inflammation.
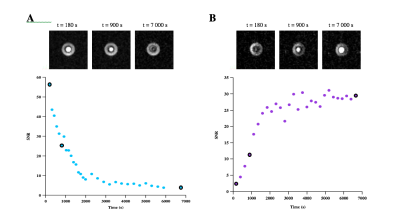
Finally, specific irradiation of the substrate and/or product frequency was swept by tuning the cavity of the OMRI setup. Maximum signal enhancements were observed at distinct electronic EPR frequencies: 5425.6 MHz for the substrate and 5414.4 MHz for the product. A maximum enhancement of 5 for the substrate was obtained at the beginning of the reaction whereas a maximum enhancement of 8 for the product was obtained at the end of the reaction (Figure 3). Thus, this substrate is suitable for the detection of NE activity by OMRI. It is also an imaging method suitable to visualize the bio-distribution of the substrate.
Conclusion
Proteolysis imaging high contrast was given by OMRI in the presence of a nitroxide. The contrast was conditioned to neutrophil elastase catalysis by linking a specific peptide to a line-shifting nitroxide. Thus, by choosing the EPR irradiating frequency of the “Overhauser switch” either the substrate or the product would produce contrast. All OMRI experiments validated the use of the substrate Meo-Suc-(Ala)2-Pro-Val-nitroxide as a reliable marker of inflammation. Michaelis constants reveal a better substrate than its optically active paranitroanilide analog. After characterization, the substrate was successfully tested on samples from a Pseudomonas aeruginosa lung infection. In mouse BALs an activity of 1 nM of NE is detected. In vivo, OMRI contrast of 200 % is expected from such results.Perspectives
The first expected impact of this study is to provide a useful and very specific diagnostic tool for inflamed lungs. More generally, the goal is to perform molecular MRI of any protease/inhibitor imbalance with 3D resolution firstly for neutrophil elastase. This diagnosis at a molecular scale could be done at a much earlier stage of the disease, prior to any anatomical alteration. Ultimately it could be used as a monitoring tool for a personalized treatment.Acknowledgements
This study was achieved within the context of the ANR PULMOZYMAGE (ANR-15-CE18-0012-01) and the Cluster of Excellence TRAIL ANR-10-LABX-57. The authors thank Aix-Marseille University for A*MIDEX grant (ANR-11-IDEX-0001-02) funded by the Investissements d’Avenir French Government program, managed by the French National Research Agency (ANR). Thanks also to “Fonds Agir pour les Maladies Chroniques”, Rhône Alpes Auvergne.References
1. B. Korkmaz, M. S. Horwitz, D. E. Jenne, F. Gauthier, Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacological reviews 62, 726-759 (2010); published online EpubDec (10.1124/pr.110.002733).
2. N. Guyot, J. Wartelle, L. Malleret, A. A. Todorov, G. Devouassoux, Y. Pacheco, D. E. Jenne, A. Belaaouaj, Unopposed cathepsin G, neutrophil elastase, and proteinase 3 cause severe lung damage and emphysema. The American journal of pathology 184, 2197-2210 (2014); published online EpubAug (10.1016/j.ajpath.2014.04.015).
3. G. Audran, L. Bosco, P. Bremond, J. M. Franconi, N. Koonjoo, S. R. Marque, P. Massot, P. Mellet, E. Parzy, E. Thiaudiere, Enzymatically Shifting Nitroxides for EPR Spectroscopy and Overhauser-Enhanced Magnetic Resonance Imaging. Angewandte Chemie 54, 13379-13384 (2015); published online EpubNov 02 (10.1002/anie.201506267).
4. N. Jugniot, I. Duttagupta, A. Rivot, P. Massot, C. Cardiet, A. Pizzoccaro, M. Jean, N. Vanthuyne, JM. Franconi, P. Voisin, G. Devouassoux, E. Parzy, E. Thiaudière, S.R.A. Marque, A. Bentaher, G. Audran, P. Mellet. Article submitted. An Elastase Activity Reporter for EPR and OMRI as a Line-Shifting Nitroxide
5. C. Koehl, C. G. Knight, J. G. Bieth, Compared action of neutrophil proteinase 3 and elastase on model substrates. Favorable effect of S'-P' interactions on proteinase 3 catalysts. The Journal of biological chemistry 278, 12609-12612 (2003); published online EpubApr 11 (10.1074/jbc.M210074200).
6. A. Belaaouaj, R. McCarthy, M. Baumann, Z. Gao, T. J. Ley, S. N. Abraham, S. D. Shapiro, Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nature medicine 4, 615-618 (1998); published online EpubMay.
Figures