3581
Low affinity zinc sensors for improved MRI detection of glucose-stimulated zinc(II) secretion from pancreatic beta-cells in vivo1Advanced Imaging Research Center, UTSW Medical Center, Dallas, TX, United States, 2UT Dallas, Richardson, TX, United States
Synopsis
We report here the design of several gadolinium-based MR contrast agents with different zinc affinities. The zinc sensors increased r1 in the presence of Zn2+ ions and more in the presence of serum albumin, only when zinc was present. The sensors with a lower affinity for Zn2+ enhanced better the MR contrast produced by zinc release in mouse pancreas due to reduced background signal. These lower affinity Zn2+ agents show great promise for detecting and monitoring the pharmacological effect of drugs in diabetes.
SYNOPSIS
We report here the design of several gadolinium-based MR contrast agents with different zinc affinities. The zinc sensors increased r1 in the presence of Zn2+ ions and more in the presence of serum albumin, only when zinc was present. The sensors with a lower affinity for Zn2+ enhanced better the MR contrast produced by zinc release in mouse pancreas due to reduced background signal. These lower affinity Zn2+ agents show great promise for detecting and monitoring the pharmacological effect of drugs in diabetes.INTRODUCTION
Imbalances in tissue zinc has been associated with diseases such as Alzheimer’s disease, diabetes, transient neonatal zinc deficiency, and prostate cancer.1–3 In the pancreas, zinc is packaged with crystalline insulin in beta-cell granules and co-released with insulin in response to an increase in plasma glucose. Glucose-stimulated insulin secretion (GSIS) is known to occur in two phases, a rapid release initial phase followed by a more prolonged slow release phase. Development of type 2 diabetes has been associated with a loss of the rapid release portion of insulin secretion so it would be helpful for future drug development to have an imaging method to monitor the rapid release phase of insulin secretion in real time in animals.METHODS
In this work, we have synthesized and characterized several GdDO3A-BPEN agents for zinc sensing. The biophysical properties were determined by 17O NMR and proton relaxometry at low and high field (0.4 and 9.4T). The equilibrium dissociation constants for some of the possible systems in the ternary complex equilibria were determined by fluorescent competition methods, proton relaxometry and 113Cd NMR spectroscopy. Kinetic inertness was studied by proton relaxometry in the presence of excess ZnCl2 and phosphate buffer. T1-weighted images of wild type mice were recorded in vivo at 9.4T to monitor GSIS. A complex equilibrium binding model to explain the MRI observations was written in MatLab code.RESULTS
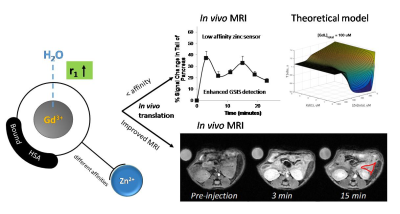
All zinc-responsive agents showed an increase in r1 relaxivity upon Zn2+ binding. Upon addition of HSA to the system, the r1 values increased even further about 4-fold (~22 s-1mM-1). By using different spectroscopic techniques, the Zn2+ ion binding affinities with each GdL sensors had KD values ranging from nM to mM. Biophysical NMR methods were used to determine the parameters governing the paramagnetic properties of the systems including the rate of water exchange (kex), molecular tumbling rates (τR), and kinetic inertness. The complexes showed optimized kex and remarkable kinetic inertness when compared to GdDTPA and other previously reported GdDOTA-diBPEN agents.4,5 T1-weighted MR images of wild-type mice showed significant contrast enhancement in the pancreas after co-injection (iv) of an agent plus glucose. Importantly, a comparison of high and low Zn2+ affinity agents showed that the low-affinity agents showed greater enhancement of the pancreas pre- versus post-glucose administration (Figure 1). A model of the dynamic equilibria that considers all possible GdL, Zn2+, and HSA species predicts that an agent with lower affinity for Zn2+ results in a lower background MR signal prior to glucose administration and this results in a greater increase in MR signal after glucose initiates release of additional Zn2+ and insulin from the pancreas.DISCUSSION
We show here that a developed weaker Zn2+ affinity agent may indeed be more sensitive to small changes in Zn2+ levels, especially at anticipated higher Zn2+ concentrations during active secretion of insulin. The in vivo MR imaging at the tail of the pancreas confirmed our hypothesis that the reduced the background signal arising from basal Zn2+ in plasma during constant infusion of a zinc low affinity agent results in better detection of the Zn2+ release from β-cells. With biophysical 113Cd NMR experiments and a theoretical model that describes all species in this dynamic ternary-complex system we could predict this behavior. The practical result of this calculation shows that the background image intensity would be considerably higher when using a high affinity Zn2+ agent. Also, the low affinity agents show a continuous detection of the zinc release from 50-600mM, whereas the high affinity sensors are only effective at changes of the zinc release below 200mM.CONCLUSION
These results demonstrate that by reducing the background signal arising from basal levels of free Zn2+ in the plasma it is possible to design and optimize functional MRI contrast agents that respond to local increases in the concentration of important biological ions in vivo. Biologically responsive MR agents such as these could have an immediate impact in the development of new diabetes drugs and could eventually provide clinical insights into disease processes that are simply not available using current clinically approved MR contrast agents.Acknowledgements
The authors acknowledge partial financial support for this work from the National Institutes of Health (CA-115531, EB-01598, EB-00482), Harold C. Simmons Cancer Center through an NCI Cancer Center Support Grant, 1P30 CA142543, and the Robert A. Welch Foundation (AT-584).References
1. De Leon-Rodriguez L, Lubag Jr. AJM, Sherry AD. Imaging free zinc levels in vivo – What can be learned? Inorganica Chim Acta. 2012;393:12–23.
2. Jordan MVC, Lo S-T, Chen S, Preihs C, Chirayil S, Zhang S, Kapur P, Li W-H, Leon-Rodriguez LMD, Lubag AJM, Rofsky NM, Sherry AD. Zinc-sensitive MRI contrast agent detects differential release of Zn(II) ions from the healthy vs. malignant mouse prostate. Proc Natl Acad Sci. 2016;113(37):E5464–E5471. PMID: 27562169
3. Lo S-T, Martins AF, Jordan VC, Sherry AD. Zinc as an Imaging Biomarker of Prostate Cancer. Isr J Chem. 2017;57(9):854–861.
4. Yu J, Martins AF, Preihs C, Clavijo Jordan V, Chirayil S, Zhao P, Wu Y, Nasr K, Kiefer GE, Sherry AD. Amplifying the Sensitivity of Zinc(II) Responsive MRI Contrast Agents by Altering Water Exchange Rates. J Am Chem Soc. 2015;137(44):14173–14179.
5. Esqueda AC, López JA, Andreu-de-Riquer G, Alvarado-Monzón JC, Ratnakar J, Lubag AJM, Sherry AD, De León-Rodríguez LM. A New Gadolinium-Based MRI Zinc Sensor. J Am Chem Soc. 2009;131(32):11387–11391.