3580
Imaging and discrimination of extracellular lactate in vivo using CEST and a paramagnetic shift reagent1Advanced Imaging Research Center, UTSW Medical Center, Dallas, TX, United States, 2UT Dallas, Richardson, TX, United States, 3Universidade da Coruña, Coruña, Spain
Synopsis
Glucose taken up by cancer cells is thought to be converted largely to lactate even in the presence of abundant oxygen although the amount of pyruvate diverted into the mitochondria is largely unknown. Hence, a method for imaging actual lactate production by tumors could be highly informative. We report in this work the design of several lanthanide-based shift reagents (SR) that form complexes with lactate and shift the lactate –OH CEST signal to a different frequency far away from water. Given that these SR’s are confined to extracellular space, the resulting lactate –OH CEST signal becomes a specific biomarker of lactate exported from cancer cells. This method offers great promise for imaging lactate production by tumors.
INTRODUCTION
Contrast agents are often used in magnetic resonance imaging (MRI) studies to enhance contrast between tissues. During the last decade, a new type of contrast mechanism based on chemical exchange saturation transfer (CEST) has been explored using a variety of diamagnetic and paramagnetic molecules.1 Many endogenous molecules containing exchangeable –OH protons have been explored as potential CEST agents, including glucose and glycogen. Lactate also has an exchangeable –OH proton that could potentially be detected by CEST but the chemical shift of the lactate –OH proton is even closer to water than the –OH protons of glucose.2 Hence, it is quite difficult to detect lactate directly using CEST activation pulses. L-lactate is overproduced by most tumors even in the presence of abundant oxygen (the Warburg effect) so a method for direct imaging of lactate production by tumors could be a useful biomarker. D-lactate, although rarely produced by human cells, is also formed by some essential bacteria from the human microbiota. Consequently, D-lactate acidosis is associated with several diseases including bowel syndrome and D-lactate encephalopathy.3,4 These data provide the framework for developing new SRs to image lactate production in tumors by MRI.METHODS
Several different tris-amide derivatives of the common macrocyclic ligand, DO3A, were prepared and characterized. The Yb3+ and Eu3+ complexes were prepared and characterized by 1D and 2D NMR, X-ray crystallography and density functional theory (DFT) calculations. The CEST profiles were recorded and the pH and temperature dependencies of the proton exchange rate in each complex (kex) was determined using the Omega fitting method. T1-weighted and CEST images of mice having a A549 small cell lung cancer xenograft tumor growing on a lower flank were recorded in vivo after injection of a lactate-specific SR.RESULTS
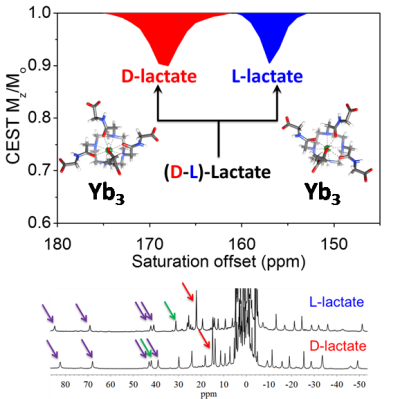
The CEST signal from lactate in aqueous buffer is largely obscured by the bulk water resonance. Addition of a lactate-specific SR to the sample resulted in a shift of the lactate –OH CEST peak well away from the water resonance, from 50-130 ppm depending upon which lanthanide complex was used.5,6 The CEST signal of the SR·lactate complex was both temperature and pH dependent. The CEST signal was most intense under more acidic conditions (pH ~ 5-6.5) and at a physiologic temperature of 310K. The lanthanide complexes were quite selective for lactate even in the presence of other competing ligands. Complexes with d-chiral side arms were capable of chiral discrimination of D- versus L-Lactate in aqueous solution. In vivo CEST images of control mice showed that an Eu-based SR co-injected with lactate (1:1) was easily detected in the mouse bladder after 30 min. In tumor-bearing mice, IV injection of the SR alone showed a signal from lactate only within certain regions of the tumor. These preliminary data suggest that one may be able to image lactate production regionally in tumors using CEST MRI and a simple paramagnetic shift reagent.DISCUSSION
We
demonstrate here that one can shift the –OH resonance of L-lactate far
downfield from tissue water protons using Eu3+ and Yb3+-based
paramagnetic shift reagent (SR) and then use the “on” versus “off” CEST
response to quantify lactate produced by tumor cells growing in tissue culture
(Figure 1). Depending upon which paramagnetic complex is used, the shifts can
be quite large (50-130 ppm), moving the CEST signal of lactate well away from
other confounding endogenous signals from tissues. Interestingly, the intensity
of the lactate CEST signal was enhanced under slightly acidic conditions
(similar to that produced by tumors) and was more intense at 37°C than at 25°C.
D- versus
L-lactate were easily distinguished based upon the frequency of their CEST
signals using a Yb3+-based SR with d-chiral
side-arms. The potential of the SR was
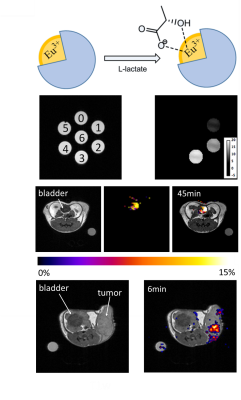
demonstrated by imaging excess lactate excreted into the bladder of a wild-type
mouse and also by detecting extracellular lactate produced in mice bearing
small cell lung cancers in vivo (Figure 1).CONCLUSION
The potential of the SR was demonstrated by imaging excess lactate excreted into the bladder of a wild-type mouse and by detecting extracellular lactate produced in a small cell lung cancer tumor model in vivo (Figure 1). These results provide the framework to develop and optimize new SRs to image lactate production in tumors by MRI.Acknowledgements
The authors acknowledge partial financial support for this work from the National Institutes of Health (CA-115531, EB-01598, EB-00482), Harold C. Simmons Cancer Center through an NCI Cancer Center Support Grant, 1P30 CA142543, and the Robert A. Welch Foundation (AT-584).References
1. Viswanathan S, Kovacs Z, Green KN, Ratnakar SJ, Sherry AD. Alternatives to Gadolinium-based MRI Metal Chelates. Chem Rev. 2010;110(5):2960–3018. PMCID: PMC2874212
2. van Zijl PCM, Yadav NN. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn Reson Med. 2011;65(4):927–948.
3. de Bari L, Moro L, Passarella S. Prostate cancer cells metabolize d-lactate inside mitochondria via a d-lactate dehydrogenase which is more active and highly expressed than in normal cells. FEBS Lett. 2013;587(5):467–473.
4. Kowlgi NG, Chhabra L. D-Lactic Acidosis: An Underrecognized Complication of Short Bowel Syndrome. Gastroenterol Res Pract. 2015;2015:e476215. PMID: 25977687
5. Zhang L, Martins AF, Mai Y, Zhao P, Funk AM, Clavijo Jordan MV, Zhang S, Chen W, Wu Y, Sherry AD. Imaging Extracellular Lactate In Vitro and In Vivo Using CEST MRI and a Paramagnetic Shift Reagent. Chem – Eur J. 2017;23(8):1752–1756.
6. Zhang L, Martins AF, Zhao P, Tieu M, Esteban-Gomez D, McCandless GT, Platas-Iglesias C, Sherry AD. Enantiomeric recognition of D- and L-lactate by CEST with the aid of a paramagnetic shift reagent. J Am Chem Soc. 2017 DOI: http://dx.doi.org/10.1021/jacs.7b08292
Figures