3579
In Vivo iCEST MRI Detection of Zinc Depletion in an Orthotopic Prostate Cancer Mouse Model1The Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
Synopsis
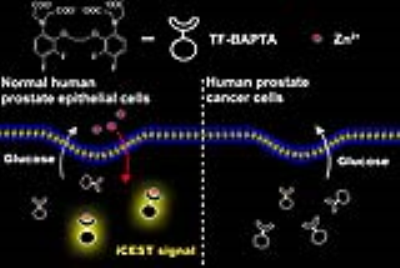
Prostate cancer is the only known disease of the prostate that displays such a substantial decrease in tissue zinc content and neither prostatitis nor benign prostatic hyperplasia are associated with this phenotype. 19F-based iCEST MRI probe TF-BAPTA was used to show clearly a detectable difference in zinc concentration between normal and malignant prostate cell lines, and normal prostate cells with a downregulated ZIP1 transporter. Via an orthotopic prostate cancer mouse model, the feasibility of iCEST MRI to distinguish normal mouse prostate and cancerous prostate has been verified. Hence, iCEST MRI may have potential to non-invasively monitor the early malignant transformation in prostate cancer.
Target Audience
Researchers, radiologists and other clinicians interested in prostate cancer imaging, 19F MRI and CEST imaging.
Purpose
The healthy prostate contains the highest concentration of mobile zinc of all soft tissues in the body. This level decreases dramatically during the development of prostate cancer1,2. Therefore, non-invasive detection of prostate zinc content may potentially be applied for an early diagnosis of malignant transformation. A new approach is to use 19F MRI with fluorinated probes, termed iCEST, which is capable to detect sub-millimolar Zn2+ concentrations3. Here, we show that the iCEST MRI is able to differentiate between normal and malignant prostate cells (as outlined in Figure 1) and to detect zinc deficiency in vivo in an orthotopic prostate cancer mouse model.Methods
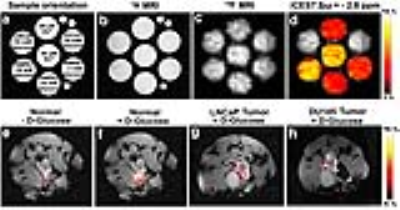
Normal (RWPE1), ZIP1 zinc transporter-downregulated (RWPE2), and malignant (DU145 and LNCaP) prostate cells were incubated with 75 μM ZnSO4 for 72 h and then washed with secretion assay buffer for 3 times. Induction of zinc secretion by glucose stimulation was carried out according to a previous report2. After centrifugation, cells were divided equally into three wells, and co-incubated with 2.5 mM 5,5’,6,6’-tetrafluoro-1,2-bis(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid (TF-BAPTA) and 0, 3, or 18 mM glucose for 1 h, respectively. The supernatant of cells was collected in 5 mm NMR tubes. MRI experiments were performed on a Bruker 17.6 T scanner with vertical bore. For 19F iCEST MRI, the center frequency was set at the frequency of the 19F atom at the 6 position (0.0 ppm) of TF-BAPTA. A modified RARE sequence (TR/TE=3,053/2.87 ms, RARE factor=16, 8 mm slice, FOV=1.6×1.6 cm, matrix size=32×32, resolution=0.5×0.5 mm, average number=96 and a saturation pulse B1=2.4 µT/3 s) was used. 1 x 106 cancer cells were injected into the prostate of 6-8 weeks old immunodeficient NSG mice and allowed to grow for 21 days. All normal NSG mice and orthotopic tumor-bearing NSG mice were fasted for 12 h before the iCEST MRI procedure. Immediately after intraperitoneal (i.p.) injection of 80 μL of 20% w/v D-glucose and injection of 0.15 g/kg bw TF-BAPTA into the anterior prostate through a catheter, iCEST MRI images were collected. A modified RARE sequence was used with TR/TE=3,053/2.87 ms, RARE factor=16, 3 mm slice thickness, FOV=2.6×2.6 cm, matrix size=32×32, resolution=0.8×0.8 mm, average number=8 and a saturation pulse B1=2.4 µT/3 s.Results
In vitro, the strongest iCEST signal was observed for glucose-stimulated RWPE1 cells with a normal zinc transporter level (Figure 2d). In normal prostate cells with a downregulated ZIP1 zinc transporter (RWPE2), a weaker iCEST signal was observed. No signal could be observed for DU145 and LNCaP prostate cancer cells. The concentration of released zinc from RWPE1 cells stimulated by 3 mM and 18 mM glucose was calculated to be approximately 20 μM and 40 μM, respectively. In vivo, the strongest iCEST signal was observed for the normal prostate following i.p. glucose stimulation (Figure 2f). Both the normal prostate without glucose stimulation and the two orthotopic tumor models with glucose stimulation showed much weaker iCEST signal.Conclusions
Using iCEST MRI, differences in glucose-induced zinc secretion between normal and malignant prostate cells can be readily detected, both in vitro and in vivo. The feasibility of serially monitoring the conversion of normal prostate cells into malignant cells is now further being evaluated in a transgenic adenocarcinoma of the mouse prostate (TRAMP) model.Acknowledgements
This project was supported by R03 EB018882 and the Pearl and Yueh-Heng Yang Foundation.References
1Ghosh, S. K. et al. Cancer Res. 2010, 70, 6119. 2Clavijo Jordan, V. et al. Proc. Natl. Acad. Sci. 2016, 113, E5464. 3Bar-Shir, A. et al. J. Am. Chem. Soc. 2015, 137, 78.Figures