3578
Intracellular Assembly of Olsalazine Nanoparticles for CEST MR Imaging and Cancer TherapyYue Yuan1, Xiaoliang Qi1, Jia Zhang1, Xiaolei Song1, Michael T. McMahon2, and Jeff W.M. Bulte1
1The Russell H. Morgan Department of Radiology and Radiological Science; Cellular Imaging Section, Institute for Cell Engineering, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
Synopsis
Based on a biocompatible condensation reaction caused by tumor-related proprotein convertase furin, we developed a novel probe RRVR-Olsa for tumor-targeted CEST MR imaging and chemotherapy to accomplish theranostics. Our preliminary studies indicated that, RRVR-Olsa elicited an obvious increase in CEST signal and higher cytotoxicity on furin-overexpressing HCT116 cancer cells than on furin-deficient LoVo cells and on furin inhibitor-treated HCT116 cancer cells. In vivo CEST MRI with the use of RRVR-Olsa could readily distinguish the difference of furin expression in HCT116 tumor and LoVo tumor, which we attributed to the furin-directed intracellular self-assembly of RRVR-Olsa to olsalazine nanoparticles with enhanced accumulation.
Target Audience
Researchers, radiologists and other clinicians interested in tumor-targeted imaging, cancer therapy, and CEST imaging.Purpose
We developed a new molecular probe termed “RRVR-Olsa” (Fig. 1a), in which the exchangeable hydroxyl protons in olsalazine provide chemical exchange saturation transfer (CEST) contrast with the cell-penetrating peptide RRVR acting as a specific substrate for furin1. After the RRVR-Olsa enters furin-expressing cells, the peptide is cleaved by furin, initiating a condensation reaction between the GSH-induced 1,2-aminothiol group and the cyano group of the 2-cyanobenzothiazole motif2-3, thereby resulting in the formation of olsalazine nanoparticles (Olsa-NPs) to enhance CEST signal. As olsalazine is an effective drug for colon cancer4, we investigate whether intracellular assembly of Olsa-NPs could represent a new theranostic approach.Methods
CEST imaging: Furin-overexpressing HCT116 human colon cancer cells and furin-deficient (control) LoVo human colon cancer cells were incubated with or without 150 µM RRVR-Olsa for 24 h, and then cells were washed and collected in 5 mm NMR tubes. Cells were mixed with 2% agarose to prevent sedimentation. A modified RARE sequence (TR/TE=6,000/5 ms, RARE factor=32, 1 mm slice thickness, FOV=14×17 mm, matrix size=64×64, resolution=0.22×0.27 mm, NA=2, and a saturation pulse B1=3.6 µT/4 s) was used. For in vivo tumor-targeting studies, each nude mouse (NU/J from Jackson, female, 6-8 weeks) was subcutaneously injected with 1x106 LoVo cells in the left thigh and 1x106 HCT116 cells in the right thigh. When the tumor reached a size of 5-8 mm, mice were injected i.v. with 0.1g/kg bw RRVR-Olsa, and CEST MRI was performed after 1.5 h on a 11.7 T Bruker horizontal scanner. A modified RARE sequence (TR/TE=5,000/3.7 ms, RARE factor=23, 1 mm slice thickness, FOV=3×3 cm, matrix size=64×64, resolution=0.48×0.48, NA=2 and a saturation pulse B1=3.6 µT/3 s) was used. Cell viability: The number of cells was counted after incubation with RRVR-Olsa or olsalazine at different concentrations (125, 250, and 500 µM) for 48 h (HCT116 cells), after co-incubation of 250 µM RRVR-Olsa and furin inhibitor (HCT116 cells), and after incubation with 250 µM RRVR-Olsa (LoVo cells). Cell viability was calculated as a percentage of control (untreated) set at 100%. Data were expressed as means ± SD from three independent experiments.Results
Fig. 1b clearly shows an enhanced CEST MRI contrast for furin-overexpressing HCT116 cells compared to furin-deficient LoVo cells and furin inhibitor-treated HCT116 cells, implying that the furin-catalyzed intracellular formation of Olsa-NPs is accountable for the signal enhancement. Cell toxicity was observed for incubation with 125 µM RRVR-Olsa, but not for free olsalazine that showed low cytotoxicity up to 500 µM. A comparable cytotoxicity of free olsalazine vs. RRVR-Olsa did not occur until the drug concentration was increased to 2.5 mM (Fig. 2). Furthermore, RRVR-Olsa exhibited much lower cell viability in HCT116 cells than in LoVo cells and the furin inhibitor-treated HCT116 cells, indicating that the cytotoxicity is induced by the furin-dependent self-assembly of Olsa-NPs. At 1.5 h after i.v. injection of RRVR-Olsa, furin-overexpressing HCT116 tumors and low furin-expressing LoVo tumors could be clearly distinguished from each other in vivo on CEST MRI (Fig. 3).Discussion
After a condensation reaction, olsalazine nanoparticles are formed which accumulate inside furin-overexpressing HCT116 cells. Its prolonged olsalazine drug release properties enhances its cytotoxicity compared to free olsalazine. Through the specific intracellular interaction with furin, the accumulation of RRVR-Olsa enables in vivo CEST MRI differentiation between furin-overexpressing and furin low-expressing tumors. Hence, RRVR-Olsa may find applications as a new theranostic probe.Acknowledgements
This project was supported by the Pearl and Yueh-Heng Yang Foundation.References
1. Bassi, D. E.; et al. Mol. Carcinogen. 31. 224-232 (2001). 2. Yuan, Y.; et al. Angew. Chem. Int. Ed. 54. 9700-9704 (2015). 3. Yuan, Y.; et al. ACS Nano 54. 761-768 (2015). 4. Brown, W. A.; et al. Digest. Dis. Sci. 45. 1578-1584 (2000).Figures

Fig. 1 (a) Chemical structure of RRVR-Olsa and schematic
illustration of intracellular furin-controlled self-assembly
of Olsa-NPs. (b) MTRasym curves and CEST MR images (from top to bottom) of HCT116
cells treated with 150
µM RRVR-Olsa, LoVo cells treated with 150 µM RRVR-Olsa, HCT116 cells cotreated
with 150 µM RRVR-Olsa
and furin inhibitor, and untreated HCT116 cells and LoVo cells.
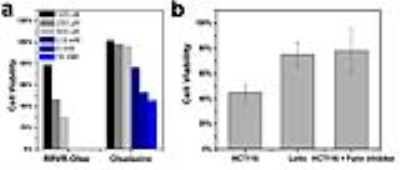
Fig. 2 (a) Cell viability of HCT116 cells incubated
with RRVR-Olsa and olsalazine at different concentrations for 48 h and (b) effects of RRVR-Olsa (250 µM) on the
cell viability of HCT116 cells, LoVo cells, and furin inhibitor-treated HCT116
cells.
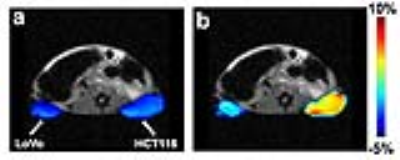
Fig.
3 In vivo CEST MR images (at 9.8
ppm offset from water) acquired (a) before
and (b) 1.5 h after i.v. injection of
0.1 g/kg RRVR-Olsa into tumor-bearing nude mice.