3576
Macrocyclic Xenon hosts: potential inhibitors and reporters for protein aggregation in Hyper-CEST MRI1Molecular Imaging, Leibniz Forschungsinstitut für Molekulare Pharmakologie, Berlin, Germany
Synopsis
Contrast agents for neurodegenerative diseases such as amyloidosis are challenging for MRI due to sensitivity limitations. Using hyperpolarized Xenon with special Xe hosts in combination with chemical excitation saturation transfer (CEST) has the potential to overcome this issue. It has been demonstrated that Cucurbit[7]uril (CB7) is inhibiting the fibrillation of Aβ40/ Aβ42 by interaction with its Phe residues. In parallel, we have demonstrated that CB7 can be used as a Xe-host. Here we present first results for a concept that uses CB7 as a drug and Xe biosensor simultaneously where binding of CB7 to Aβ40 can be detected by changing the Xe-HyperCEST signal.
Introduction
Contrast agents for neurodegenerative diseases such as amyloidosis of Aβ or α-synuclein are challenging to implement for MRI due to the low target concentration in vivo. Therefore, the method of choice for diagnosis so far is the application of positron emission tomography (PET), which makes the unfavourable use of radiotracers necessary.
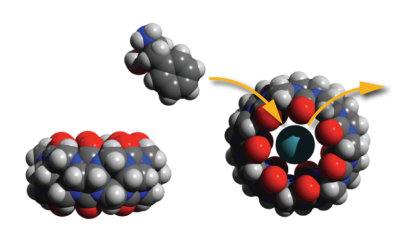
In this context it is remarkable that it has been shown that the molecular container Cucurbit[7]uril (CB7) does inhibit Aβ 40 as well Aβ 42-peptide fibrillation effectively 1. It seems this effect relies upon binding of CB7 to the side chains of phenylalanine residues (see Fig. 1) and thereby preventing the hydrophobic interaction of these residues, which trigger amyloid fibrillation. We have recently investigated the Xe-binding capabilities of CB7 and successfully used it as a sensor for hyperpolarized xenon in chemical exchange saturation transfer (CEST) magnetic resonance imaging (MRI) 2. Xenon biosensors in general opened up an unprecedented sensitivity to MRI down to nanomolar concentrations and even below by combination with CEST 3-5. This taken together with the selectivity of CB7 for Aβ-peptides is opening the pathway for a potentially new xenon biosensor, which can be used as a drug at the same time. Our approach is also promising in the context of the recently reported successful in vivo application of CB6, a closely related member in the group of Cucurbiturils 6.
Results
We investigated the interaction of CB7 with Aβ40 in a xenon HyperCEST experiment. In order to guarantee monomeric peptides during the duration of initial experiments the spectra have been recorded in 1,1,1,3,3,3- Hexafluoro-2-propanol (HFIP). We observed indeed that Aβ40 is blocking the cavity of CB7 and switches off the initially detected HyperCEST signal of CB7 (see Fig. 2) by preventing xenon to interact with the cavity. The effect occurs already for equimolar ratios of CB7: Aβ40.
These promising results serve as a starting point for designing a tandem biosensor that uses loss of the CB7 CEST-effect upon binding to the Aβ-like peptides in combination with CrA as an always-on CEST xenon biosensor. This would enable us to image amyloidosis as well as serving as a potential treatment by CB7 while detecting where accumulation of the biosensor takes place.
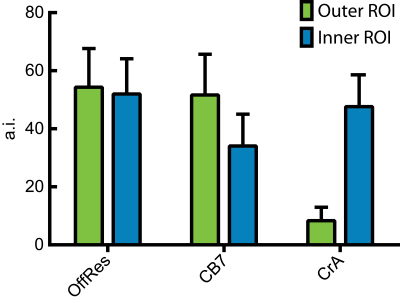
As a prerequisite for this, we optimized the pulse sequence and saturation powers to enable the selective detection of each xenon hosts. The challenge is to find the balance between a CEST response from CB7 without too much cross talk to the signal from CrA. Both signals are separated by ca. 42 ppm, i.e. ca. 4663 Hz. The CB7 response is characterized by faster exchange of the Xe atoms and therefore requires stronger saturation pulses to generate image contrast. A side effect of stronger saturation pulses is that they affect a broader region in the spectral domain. Hence, care has to be taken to avoid too much response from CrA-associated Xe while directly affecting the Xe bound to CB7 and vice versa. We therefore chose to work with somewhat moderate pulses but apply them for a sufficiently long saturation time still achieving a measurable image contrast. Overall, we could observe an average signal loss of 35% for saturating at the CB7 resonance and of 93% for addressing the CrA signal. Fig. 3 shows the respective 129Xe MRI scans. This data clearly demonstrates for the first time that it is possible to selectively switch on Xe MRI contrast from either CB7 or CrA within the same setup. The analysis of two different regions of interest (ROIs) covering the two compartments is shown in Fig. 4. The unwanted response of the other molecule while selectively addressing either CB7 or CrA remains below 10%.
Conclusion
We identified suitable encoding conditions for MRI of dissolved Xe (which is ca. 1 million-fold lower in spin density than water protons in conventional MRI) and demonstrated that optimized encoding yields appropriate image quality in realistic scanning time. Due to the different exchange behavior of Xe in either CrA or CB7, balanced saturation transfer conditions were identified to retain spectral sensitivity when implementing the tandem reporter containing both host types in the future. With this at hand together with our findings of Phe binding to CB7 we are confident in about establishing an MRI biosensor for detecting protein aggregation.Acknowledgements
The Michael J. Fox Foundation is acknowledged for funding (Grant ID 12549).References
1. Lee, H. H. et al.; Supramolecular Inhibition of Amyloid Fibrillation by Cucurbit[7]uril; Angew. Chem. Int. Ed. 53, 7461–7465 (2014).
2. Schnurr, M., Sloniec- Myszk, J., Döpfert, J., Schröder, L. & Hennig, A.; Supramolekulare Assays zur Lokalisation von Enzymaktivität durch Verdrängungsinduzierte Änderungen in der Magnetisierungstransfer-NMR-Spektroskopie mit hyperpolarisiertem 129Xe; Angew. Chem. 127, 13645-13648 (2015).
3. Klippel, S. et al.; Cell Tracking with Caged Xenon: Using Cryptophanes as MRI Reporters upon Cellular Internalization; Angew. Chem. Int. Ed. 53, 493-496 (2013).
4. Klippel, S., Freund, C. & Schröder, L.; Multichannel MRI Labeling of Mammalian Cells by Switchable Nanocarriers for Hyperpolarized Xenon; Nano Lett. 14, 5721–5726 (2014).
5. Witte, C. et al.; Live-cell MRI with Xenon Hyper-CEST Biosensors Targeted to Metabolically Labeled Cell- Surface Glycans; Angew. Chem. Int. Ed. 54, 2806–2810 (2015).
6. Hane, F.T. et al. In vivo detection of cucurbit[6]uril, a hyperpolarized xenon contrast agent for a xenon magnetic resonance imaging biosensor; Sci. Rep., 7, 41027 (2017).
Figures