3575
Improved Hyperpolarization of Solid and Mesoporous Nanoscale Silicon Particles Using TEMPO Radicals Allows In Vivo 29Si MRI1The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 2Rowan University, Glassboro, NJ, United States, 3Rice University, Houston, TX, United States, 4Hanyang University, Ansan, Republic of Korea
Synopsis
Hyperpolarized silicon microparticles have been previously demonstrated as in vivo MRI contrast agents; unfortunately, their large size and decreased mobility present limitations for targeted molecular imaging. While nanoscale silicon particles can also be hyperpolarized, their signal enhancement is typically limited by a low concentration of endogenous electrons. As such, no studies to date have demonstrated in vivo 29Si MRI of hyperpolarized nano-scale silicon. We demonstrate improved 29Si hyperpolarization with the addition of an exogenous radical species to both solid and mesoporous nanoparticle samples (30-300 nm diameter), which increases 29Si hyperpolarization and allows in vivo imaging of silicon nanoparticles.
Introduction
Nano-scale silicon particles have potential utility as targeted molecular imaging agents due to their biocompatibility and simple surface chemistry that is amenable to drug loading and targeting [1,2]. Given that 29Si is MR-active (I=1/2), silicon nanoparticles (SiNPs) may be developed as in vivo MR contrast agents. Indeed, the ability to perform in vivo 29Si MRI has been demonstrated in silicon microparticles that are hyperpolarized using dynamic nuclear polarization (DNP) [3,4]. Unfortunately, due to their large size and lack of mobility, micro-scale silicon particles have limitations for targeted molecular imaging, especially when vascular delivery is needed [5]. While some preliminary studies have shown that nano-scale silicon particles can also be hyperpolarized via DNP [5-7], there have been no demonstrated in vivo studies to date. Indeed, in a direct comparison, nanoscale silicon particles have been shown to provide a fraction of the HP 29Si MR signal (~30x less) compared to microscale particles [5]. Here we report our efforts to improve DNP of nanoscale silicon particles through the addition of an exogenous radical species ((2,2,6,6-Tetramethylpiperidin-1-yl)oxyl, or ‘TEMPO’), which provides additional free electrons to improve the efficiency of 29Si DNP. Initial electron spin resonance (ESR) studies showed that microscale particles contained nearly 10x more available electrons compared to the nanoscale particles; this is likely due to an increase in internal defects and amorphous regions of the micro-scale particles. By titrating in different concentrations of TEMPO, we were able to maximize the achievable 29Si MR signal, thus allowing for in vivo imaging of nanoscale silicon particles. Furthermore, we also applied the addition of TEMPO to interrogate mesoporous silicon nanoparticles, which could be further developed as drug delivery theranostic agents.Methods
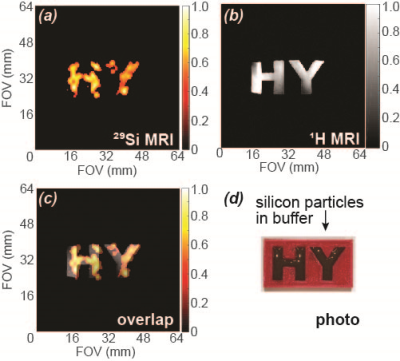
Solid silicon particles ranging from 30 to 200 nm diameter were commercially procured and used as received. Mesoporous silicon particles were synthesized using a modified Stober method to create silica particles, followed by reduction with Mg at high temperatures and pressures; this resulted in ~300 nm diameter spherical mesoporous SiNPs with ~10 nm diameter pores. These mesoporous SiNPs were surface-functionalized with 3-aminopropyl-triethoxysilane (APTES). Particle samples (~50 mg) were dissolved in a 1:1 mixture of D2O and DMSO, with varying concentrations of TEMPO radical mixed in (total sample volume ~130 µL). The particles were hyperpolarized using a laboratory-constructed 29Si solid-state DNP device [5]; imaging and spectroscopy studies were performed at room temperature on a 7T small animal MRI. Co-registered [1H:29Si] MRI was performed using a dual-tuned 29Si/1H Litz coil: in vivo 29Si imaging used a coronal RARE sequence (α = 90°; TR/TE: 60 ms/1.8 ms; 6.4 cm FOV; 2 mm resolution), while 1H imaging utilized a coronal RARE scan (α = 90°), TR/TE: 1927 ms/9.5 ms with a RARE factor of 8; 6.4 cm FOV (0.25 mm resolution) and 4 averages. 29Si spectroscopy utilized a variable tipping angle pulse to interrogate the hyperpolarization buildup or decay values.Results
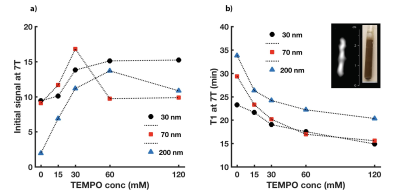
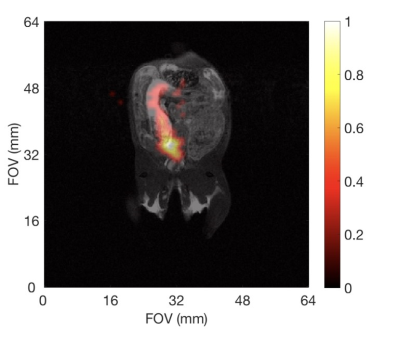
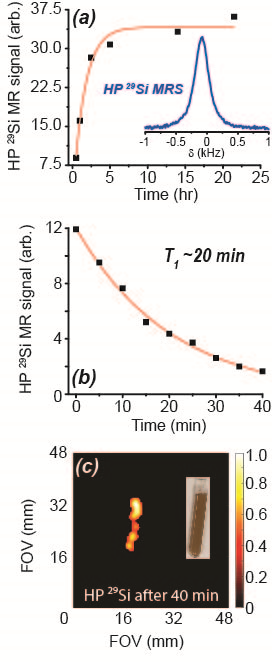
ESR studies show that adding TEMPO to the nanoparticle solutions significantly improved the pool of available free electrons for DNP. This translated to increased 29Si MR signals following DNP with added radical (Figure 1); these improvements ranged from 1.5x to more than 6x compared to DNP without TEMPO. Adding radical slightly hastened 29Si T1 decay; under conditions of optimal TEMPO concentration, T1 values decreased by ~25% for all nanoparticle sizes. Despite this faster T1, 29Si MR signal was sufficiently improved to warrant inclusion of TEMPO (i.e., increases in 29Si hyperpolarization overcame losses from T1). This allowed, for the first time, in vivo 29Si MRI of hyperpolarized nano-scale silicon particles (Figure 2), as well as studies of mesoporous silicon nanoparticles (Figures 3-5).Discussion
The ability to considerably improve 29Si hyperpolarization in nanoscale solid and mesoporous particles through the addition of exogenous radicals is a significant step forward in their development as targeted imaging agents. We present the first demonstration of in vivo imaging of nanoscale silicon particles, as well as the first reported hyperpolarization studies involving mesoporous silicon nanoparticles. The added TEMPO was not removed prior to injection into the mouse models, and no ill effects were noticed in the mice. The nanoscale silicon particles with added TEMPO performed favorably compared to microscale silicon control samples, and 29Si T1 values for the nanoparticle samples with TEMPO varied from 15-35 minutes. High resolution 29Si MR images could be achieved over 65 minutes post-DNP.Conclusions
We demonstrate that adding exogenous radicals to nano-scale silicon particles significantly improves their hyperpolarization characteristics to allow in vivo 29Si MRI. Future studies will attach targeting agents and therapeutic drugs to allow multiplexed theranostics using nanoscale silicon.Acknowledgements
Acknowledgements: This work was funded by the MDACC Odyssey Postdoctoral Fellowship, NCI R25T CA057730, DoD PC131680, MDACC Institutional Research Grants, MDACC Institutional Startup, U54 CA151668, Leukemia and Brain SPORE Developmental Research Awards, NCI R21 CA185536, Gulf Coast Consortium, CPRIT RP150701, and NCI Cancer Center Support Grant CA016672.References
[1] Park, et al. Nat. Mat. (2009)
[2] Tasciotti, et al. Nat. Nano. (2008)
[3] Cassidy, et al. Nat. Nano. (2013)
[4] Dementyev, et al. Phys. Rev. Lett. (2008)
[5] Whiting, et al. J. Med. Imag. (2016 )
[6] Atkins, et al., ACS Nano (2013)
[7] Kwiatkowski, et al., Sci. Rep. (2017).
Figures