3574
Feasibility of Imaging Lung Cancer Using Hyperpolarized MRI TechnologyMehrdad Pourfathi1, Luis Loza1, Stephen Kadlecek1, Ian Duncan1, Diane Lim2, Shampa Chatterjee3, Kai Ruppert1, Sarmad Siddiqui1, Harrilla Profka1, Yan Liu2, Jessica Kim2, Hooman Hamedani1, Yi Xin1, Faraz Amzajerdian1, Maurizio Cereda4, Ryan Baron1, Mary Spencer1, Tahmina Achekzai1, Jose Conejo-Garcia5, and Rahim R. Rizi1
1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Sleep Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3Physiology, University of Pennsylvania, Philadelphia, PA, United States, 4Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, PA, United States, 5Moffitt Cancer Center, Tampa, FL, United States
Synopsis
We demonstrate the feasibility of hyperpolarized MRI technology to image lung cancer in mice. We demonstrated the use of 13C MRSI to detect elevated pyruvate to lactate conversion in the tumor relative to the adjacent non-cancerous lung tissue. We also showed the feasibility of 129Xe imaging to detect non-aerated regions in the lung tissue co-localized with the tumor. The utility of these modalities combined may provide a multi-faceted tool to assess tumor's stage and its response to therapy in lung cancer.
Introduction
CT
and PET/CT are the most commonly-used imaging techniques for lung cancer
screening (1). However, repeated scans for staging and monitoring
tumor response to treatment expose patients to hazardous ionizing radiation. Hyperpolarized
MRI is a set of novel imaging methods that have been used safely in humans: 13C MRI to monitor treatment in
prostate cancer (2), and 129Xe to
characterize altered lung structure and function in a variety of conditions. In
this study, we demonstrate the utility of both techniques for detecting changes
in tumor metabolism and lung function, respectively, in a murine lung cancer
model.Materials and Methods
Tumor was induced in C57BL/6 mice as previously described (3). Mice were scanned in a 9.4T vertical-bore micro-imaging MRI system (Bruker Inc.) using either a dual-tuned 1H/13C or 1H/129Xe resonator (Bruker Inc.). T2-weighted images were acquired using a dual-echo respiratory gated RARE pulse sequence (TR/TE1/TE2 = 570ms, 2.1ms, 10.2ms, ETL = 4, NA = 8, matrix size = 192x192, FOV = 30x30 mm2, 0.8mm slice thickness, 16 slices. For carbon experiments, 22μL of [1-13C] pyruvate was hyperpolarized using a HyperSense DNP polarizer (Oxford Instruments HyperSense) and melted rapidly at 180oC to yield a neutral isotonic solution of 80mM hyperpolarized pyruvate. 200μL of the solution was injected via the tail vein within 3 seconds. Data acquisition started 10 seconds after the start of injection, using an 2D FID-CSI pulse sequence (TR/TE = 28/0.5ms, spectral bandwidth = 6kHz, FA = 9o, matrix size=16x16, FOV = 25x25cm2, 5mm slice thickness). For 129Xe experiments, enriched 129Xe gas was hyperpolarized via optical pumping (XeMed XeBox). Xenon Images were acquired using a respiratory-gated coronal multi-slice FLASH pulse sequence (TR/TE = 100/0.8ms, FA = 60o, matrix size = 64x64, FOV = 35x25 mm2, 2.5mm slice thickness, 4 slices) while the mouse was freely breathing a mixture of hyperpolarized 129Xe (30%), oxygen (20%) and nitrogen (50%).Results and Discussion
Figure 1 shows the T2-weighted image, 13C spectroscopic image and corresponding pyruvate and lactate maps overlaid on the T2 scan acquired from a single mouse, three weeks after the induction of cancer in the left lung. Enhanced lactate signal is co-localized with the tumors (white arrows) in two locations: over the primary tumor in the lung, as well as the secondary tumor protruding out of the thoracic cavity. The right panel shows the spectrum averaged over the healthy lung tissue (voxel A), primary lung cancer in the lung (voxel B) and a secondary tumor (voxel C). The lactate-to-pyruvate ratio derived from these spectra is elevated in the tumor (0.82 for the primary, 2.63 for the secondary tumors) relative to the adjacent normal lung tissue (Lac/Pyr = 0.32). This suggests that hyperpolarized carbon-13 MRI has the capacity to metabolically differentiate the tumor from the neighboring non-cancerous lung tissue. Figure 2 shows the T2-weighted scan in another mouse two weeks after induction of cancer in the left lung. The hyperpolarized 129Xe gas image shows the absence of ventilation in an area co-localized with the tumor. Further study is needed to characterize cancer-related changes in lung function and gas uptake by measuring the dissolved phase of 129Xe around the tumor. While this data is only preliminary, it nevertheless suggests the feasibility of using both techniques in combination as a multimodality approach to studying lung cancer. The advantage of these techniques in a clinical setting is the absence of ionizing radiation, which makes repeated scanning of patients possible.Conclusions
In this study, we demonstrated the feasibility of using hyperpolarized 13C and 129Xe imaging to investigate tumor glycolysis and altered lung function in a murine cancer model, and showed that both techniques have the ability to detect small tumors. In future studies, we intend to investigate the utility of both techniques as a multi-parametric approach for obtaining information about both lung function around the tumor and metabolism in the tumor, with the aim of improving staging and treatment response monitoring.Acknowledgements
No acknowledgement found.References
1. Schaefferkoetter JD et al, JNM2017;58:399–405.
2. Aggarwal R et al European Urology 2017
3. Sheen MR et al, Open Life Sciences 10:85
Figures
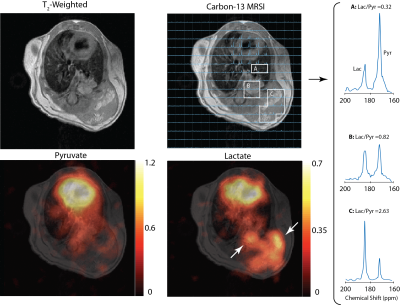
Figure 1. T2-weighted axial scan over the
lungs, showing the primary tumor in the lung and the secondary tumor protruding
out of the thoracic cavity. Metabolite maps are overlaid on the T2
scan, and the lactate map shows enhanced lactate signal over both primary and
secondary tumors (white arrows). 13C spectra averaged over the
non-cancerous lung tissue next to the tumor (A), the primary tumor (B) and the
secondary tumor (C) are shown on the right. The spectra show elevated
lactate-to-pyruvate ratio over the tumors relative to the lung tissue.
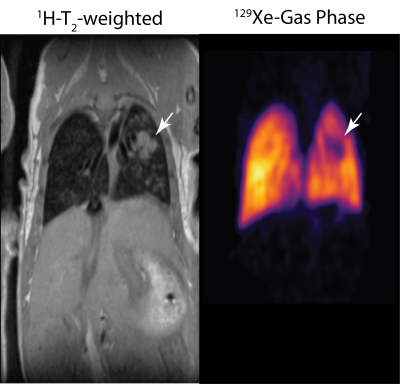
Figure 2. (Left) Coronal T2-weighted
axial scan over the lungs shows the primary tumor in the lung (white arrow).
(Right) image obtained from the airways using 129Xe gas phase
imaging shows void of signal in the area co-localized with the tumor,
suggesting absence of ventilation.