3573
Considerations for Spin Order Transfer to 13C-labeled pyruvate precursors by Parahydrogen-induced Polarization for in vivo applications1Graduate School of Information Science & Technology, Hokkaido University, Sapporo, Japan
Synopsis
The recent achievement of 13C-pyruvate polarization by side-arm para-hydrogen induced polarization (SA-PHIP) in-vitro has renewed interest in PHIP, which has been limited by a lack of biologically-relevant directly-polarizable compounds. To investigate the achievable polarization of 13C-pyruvate by SA-PHIP for in-vivo metabolic MRI, density matrix simulations of polarization transfer by magnetic field cycling (MFC) and spin-order transfer (SOT) pulse sequences were performed for target precursors. MFC-based approaches were confirmed to be suitable for polarization transfer over the long-range J-couplings present in SA-PHIP precursors. Additionally, simulated polarization levels with SOT approaches were reasonable for representative 13C-pyruvate precursors, promising for metabolic MRI.
Introduction
Parahydrogen-induced polarization (PHIP) exploits the innate spin order of the parahydrogen singlet state to generate high 1H nuclear polarization on compounds with a double bond by hydrogenation. This polarization can be subsequently transferred to nearby 13C nuclei by magnetic field cycling (MFC)1 or spin order transfer (SOT)2,3 pulse sequences. Simulated achievable 13C polarizations for compounds that can be directly polarized by PHIP can be close to unity4, however the biological relevance of these compounds is limited and their application has been restricted to angiography studies5,6.
13C-labeled pyruvate, a promising metabolic imaging target7, can be polarized directly by dynamic nuclear polarization, which is expensive to implement and polarization transfer is lengthy relative to PHIP. Recently, side-arm PHIP (SA-PHIP) has been introduced as a means to generate hyperpolarized 13C-pyruvate by polarization of a tailored precursor which is subsequently hydrolyzed to yield the polarized metabolite8. Nevertheless, sufficient 13C polarization of target precursors for in-vivo application of SA-PHIP-polarized pyruvate has not been achieved. Furthermore, only MFC-based approaches have been considered for SA-PHIP compounds; use of SOT sequences has not been revisited.
The purpose of this work was to implement density matrix simulations and compare the efficacy of PHIP SOT sequences and MFC for several conditions, and thus to investigate the obtainable polarization of 13C in suitable precursors for SA-PHIP in-vivo 13C-pyruvate metabolic MRI.
Methods
Simulations of 1H to 13C spin order transfer by MFC – as recently studied by Reineri and colleagues8,9, who employed an Earth->zero->Earth field cycling scheme – and SOT pulse sequences – the refocused parahydrogen insensitive nuclei enhanced by polarization transfer (PH-INEPT+)2 sequence (B0 ~T) and a theoretically-optimized sequence developed by Goldman & Jóhannesson3 (B0 ~mT) – were performed in Matlab (R2016a, Mathworks, Natick, MA) using a full density matrix formalism. The density matrix of parahydrogen and the Hamiltonian governing evolution under heteronuclear J-couplings were defined as follows, assuming a three-spin system HH1X (1H1H13C):
$$\rho_{PH}=\frac{\hat{E}}{4}-\left(\hat{I}_{x}^{H}\hat{I}_{x}^{H1}+\hat{I}_{y}^{H}\hat{I}_{y}^{H1}+\hat{I}_{z}^{H}\hat{I}_{z}^{H1}\right)$$
$$\hat{H}=-\nu_{1H}\left(\hat{I}_{z}^{H}+\hat{I}_{z}^{H1}\right)-\nu_{13C}\hat{I}_{z}^{X}+J_{HH1}\left(\hat{I}_{x}^{H}\hat{I}_{x}^{H1}+\hat{I}_{y}^{H}\hat{I}_{y}^{H1}+\hat{I}_{z}^{H}\hat{I}_{z}^{H1} \right)+J_{HX}\left(\hat{I}_{x}^{H}\hat{I}_{x}^{X}+\hat{I}_{y}^{H}\hat{I}_{y}^{X}+\hat{I}_{z}^{H}\hat{I}_{z}^{X} \right)+J_{H1X}\left(\hat{I}_{x}^{H1}\hat{I}_{x}^{X}+\hat{I}_{y}^{H1}\hat{I}_{y}^{X}+\hat{I}_{z}^{H1}\hat{I}_{z}^{X} \right)$$
where $$$\hat{E}$$$ is the identity matrix, $$$\hat{I}_{n}^{a}$$$ are product operators, $$$\nu_{n}$$$ are Larmor frequencies, $$$J_{nm}$$$ are J-couplings. Appropriate simplifications were used for zero, low or high fields.
Initially, the achievable 13C polarization was simulated for each technique as a function of 1H-13C J-couplings. Following this, MFC and SOT sequences were applied to two candidate molecules for generating 13C-pyruvate by SA-PHIP were investigated: ethyl-pyruvate (hydrogenation product of vinyl-pyruvate), for which J-couplings were assumed to be equivalent to ethyl-propionate9; allyl-pyruvate (hydrogenation product of propargyl-pyruvate), for which J-coupling values were inferred. For comparison, although incapable of producing hyperpolarized pyruvate, the widely-studied hydroxyethylpropionate (HEP) was also simulated4.
Results & Discussion
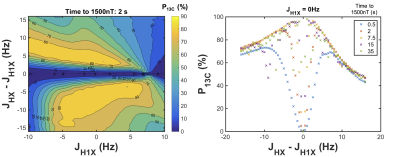
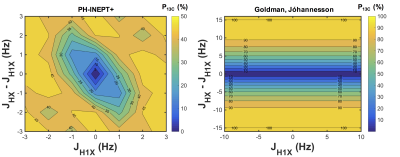
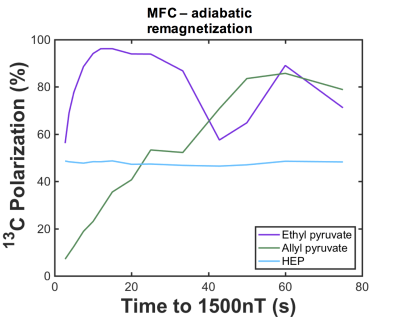
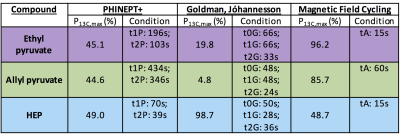
J-coupling imbalance (JHX–JH1X) is critical for optimum polarization transfer in both MFC and SOT sequence methods (chemical equivalence of the parahydrogen atoms post-hydrogenation cannot induce transfer). Figure 1 demonstrates that for MFC, slower (adiabatic) remagnetization (zero->Earth) leads to greater 13C polarization. Whilst MFC simulations showed an optimum 13C polarization for a J-coupling imbalance ~few Hz at several adiabatic remagnetization rates, both PH-INEPT+ and Goldman sequences exhibited an increasing polarization with JHX–JH1X. Figure 3 illustrates the achievable 13C polarization versus MFC adiabatic remagnetization time and Table 1 summarizes the optimum polarizations and operating conditions for MFC and SOT sequences. Although requiring a lengthier SOT sequence than HEP, ethyl- and allyl-pyruvate showed high polarization by PH-INEPT+, promising for in-vivo application. The Goldman-Jóhannesson sequence can be improved by applying additional RF pulses3; however, to achieve polarizations ~100% for ethyl-pyruvate and allyl-pyruvate, it was calculated that tens of additional pulses would need to be added; thus PH-INEPT+ presents a simpler solution. Despite some uncertainty in the J-couplings, 13C polarizations ≾1% have been achieved on allyl-acetate/pyruvate with a prototype polarizer in our laboratory (see abstract #7072, Uchio et al., ISMRM 2018).
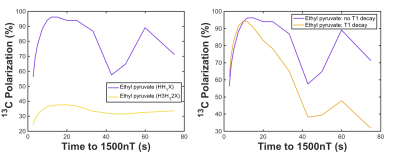
Whilst the assumption of a 3-spin HH1X system simplifies and accelerates the simulation process, in practice the surrounding (non-PH) protons also contribute to J-coupling evolution and hence the attainable polarization. Figure 4A demonstrates the effect of including these additional J-couplings for ethyl-pyruvate9. Longitudinal relaxation during SOT has been ignored to date in density matrix simulations, but becomes important in MFC for low JHX/H1X couplings where slow field cycling is required. Using an estimated T1, Figure 4b illustrates a lower-limit of the 13C polarization achieved after accounting for T1 decay.
Conclusion
Allyl- and ethyl-pyruvate may provide cost-effective means of producing hyperpolarized 13C-pyruvate by MFC or SOT pulse sequences with SA-PHIP. In particular, the simplest of the polarization transfer pulse sequences, PH-INEPT+, shows promise for obtaining reasonable 13C polarization on these precursors, and may facilitate in-vivo 13C-pyruvate metabolic MRI using an approach like that of Schmidt et al.10.Acknowledgements
NJS is an international postdoctoral research fellow of the Japanese Society for the Promotion of Science (JSPS).References
1. Jóhannesson H, Axelsson O, Karlsson M. Transfer of para-hydrogen spin order into polarization by diabatic field cycling. Comptes Rendus Phys. 2004;5(3):315-324. doi:10.1016/j.crhy.2004.02.001.
2. Haake M, Natterer J, Bargon J. Efficient NMR pulse sequences to transfer the parahydrogen-induced polarization to hetero nuclei. J Am Chem Soc. 1996;118(36):8688-8691. doi:10.1021/ja960067f.
3. Goldman M, Jóhannesson H. Conversion of a proton pair para order into 13C polarization by rf irradiation, for use in MRI. Comptes Rendus Phys. 2005;6(4-5):575-581. doi:10.1016/j.crhy.2005.03.002.
4. Bär S, Lange T, Leibfritz D, Hennig J, Elverfeldt D Von, Hövener JB. On the spin order transfer from parahydrogen to another nucleus. J Magn Reson. 2012;225:25-35. doi:10.1016/j.jmr.2012.08.016.
5. Golman K, Axelsson O, Jóhannesson H, Månsson S, Olofsson C, Petersson JS. Parahydrogen-induced polarization in imaging: Subsecond 13C angiography. Magn Reson Med. 2001;46(1):1-5. doi:10.1002/mrm.1152.
6. Bhattacharya P, Harris K, Lin AP, et al. Ultra-fast three dimensional imaging of hyperpolarized 13C in vivo. Magn Reson Mater Physics, Biol Med. 2005;18(5):245-256. doi:10.1007/s10334-005-0007-x.
7. Nelson SJ, Kurhanewicz J, Vigneron DB, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci Transl Med. 2013;5(198):198ra108. doi:10.1126/scitranslmed.3006070.
8. Reineri F, Boi T, Aime S. ParaHydrogen Induced Polarization of 13C carboxylate resonance in acetate and pyruvate. Nat Commun. 2015;6:5858. doi:10.1038/ncomms6858.
9. Cavallari E, Carrera C, Boi T, Aime S, Reineri F. Effects of Magnetic Field Cycle on the Polarization Transfer from Parahydrogen to Heteronuclei through Long-Range J-Couplings. J Phys Chem B. 2015;119(31):10035-10041. doi:10.1021/acs.jpcb.5b06222.
10. Schmidt AB, Berner S, Schimpf W, et al. Liquid-state carbon-13 hyperpolarization generated in an MRI system for fast imaging. Nat Commun. 2017;8:14535. doi:10.1038/ncomms14535.
Figures