3572
Hyperpolarization without a polarizer: in vivo 13C-MRI using SAMBADENA1Radiology - Medical Physics, University Medical Center Freiburg, Freiburg, Germany, 2Radiology and Neuroradiology, University Medical Center Schleswig-Holstein, Kiel, Germany, 3German Consortium for Cancer Research (DKTK), Heidelberg, Germany, 4German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
Hyperpolarization (HP) enhances the sensitivity of MR by several orders of magnitude and allows the detection of metabolism non-invasively and in vivo. However, well-established methods are costly, complex and require a dedicated “polarizer” next to the MRI system. SAMBADENA is, to date, the simplest and most cost-effective method to generate 13C-HP > 20 % for MRI. Within seconds HP is achieved within the MRI and little additional hardware is required. Here, the first in-vivo applications of SAMBADENA are reported, demonstrating its potential to be a fast, simple, low-cost alternative method for HP-MRI.
Introduction
The sensitivity of MRI to monitor metabolism non-invasively and in vivo can be dramatically enhanced by means of nuclear hyperpolarization (HP).1–3 Dissolution dynamic nuclear polarization (dDNP1) is well established and was used in human studies2,3, but suffers from high cost, complexity and long HP cycles. Parahydrogen (pH2)-based methods are less costly and faster but less advanced. Recently, a PASADENA (pH2 and synthesis allow dramatically enhanced nuclear alignment4) experiment was realized on a commercial MRI system: by Synthesis Amid the Magnet Bore, A Dramatically Enhanced Nuclear Alignment (SAMBADENA)5,6 with a 13C-polarization of P > 20% was achieved in vitro. Here, we present the first 13C-images acquired in vivo from SAMBADENA-hyperpolarized agents, putting the method to a real-life test with respect to the complex requirements of in-vivo imaging, namely anesthesia, animal monitoring, biocompatibility of the tracer solution (temperature, pressure) and well-controlled injection.Methods
pH2 was enriched to >90% as described elsewhere.6
Samples were
prepared as before5 using aqueous 13C-,
2H3-labeled hydroxyethyl-acrylate (HEA) at cHEA=5.5mM, 22mM or 80mM and catalyst with concentration of ccat=2mM or 4mM. pH2, was catalytically added to HEA
and 13C polarization was obtained by means of spin-order transfer
(SOT) using the sequence l-PH-INEPT+.8,9
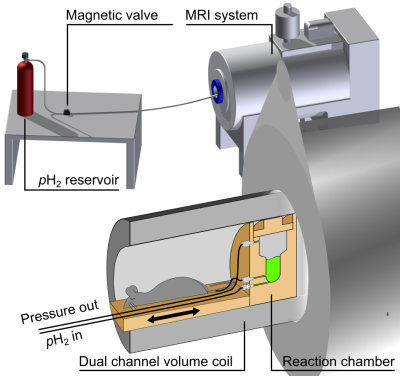
Setup: For HP and MRI a 7T
preclinical MRI system (Biospec 7/20,Bruker,Germany) was used in combination
with a dual-resonant 1H-13C-transmit-receive volume resonator
(Rapid,Germany). A high-pressure and high-temperature reactor was custom-made
to yield fast hydrogenation (inner volume ≈2ml, PSU 1000). The reactor was
mounted to a custom-built mouse bed that allows animal heating, control and
anesthesia (Fig. 1).
Hyperpolarization: The heated reactor
(≈80°C) was filled with the hot precursor solution (≈80°C), closed and moved into
the magnet. Hydrogenation was initialized by injecting pressurized pH2 (ppH2≈15-30bar) from the bottom of the reactor.
After the variable hydrogenation time th
the SOT was executed. 13C-HP was quantified assuming 100%
hydrogenation and pH2-enrichment
using a sample of thermally polarized n.a. acetone.
In-vivo
SAMBADENA: The reactor was connected to the tail vein of a mouse (30g, 14 weeks old)
that was placed next to it via a syringe using a catheter and a one-way
check-valve (IDEX, USA). An HP-tracer solution was produced and ≈300µl were injected
over a period of 10s. Another 5s later, the distribution of the tracer was
imaged using a single-shot 13C-RARE sequence (90/180°, RARE-factor: 38, FOV:
(8.4cm)2, zerofilled to 128x96px, interpolated to 256x192px, one 6cm
slice, TR=0.487s, TE=79ms, acquisition
time:0.487s). 1H-MRI was acquired for coregistration (1H-Turbo-RARE,90/180°, RARE-factor:8,
matrix:2562, TR=2.5s, TE=33ms, acquisition time:161s).
SNR was calculated as maximum intensity divided by standard deviation of the noise.
Results
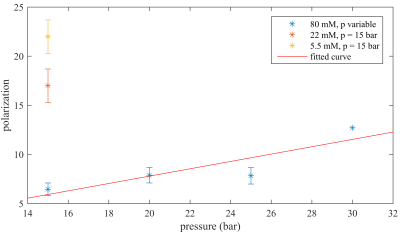
HP at high tracer
concentrations: HP was measured as function of cHEA and an inverse correlation was observed (P=(22±2)%,(17±2)% and (7±1)% at cHEA=5.5mM, 22mM and 80mM,
respectively; pH2 pressure
of 15bar; N=2 each). The experiment was repeated with 80 mM solution at ppH2=20bar,
25bar and 30bar and HP was quantified to (8±1)%, (8±1)% and 13%, respectively
(Fig. 2). Note
that the latter could not be repeated as some equipment was damaged by the
elevated pressure.
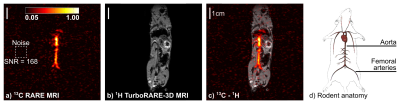
In-vivo SAMBADENA: After
the intravenous injection of HP HEP (300ml, ccat=4mM, cHEA=80mM,
P≈7 %), strong 13C
signal was imaged with an SNR of 168. The highest signal intensities originated
from the vena cava, aorta and femoral
arteries of the rodent (Fig. 3).
Discussion
For any biomedical application, highly
concentrated, highly polarized samples are needed to achieve sufficient signal in vivo. While the received signal (~cHEA*P) increased with the tracer concentration, the overall HP yield
decreased suggesting incomplete hydrogenation. As expected and discussed
before10, the HP yield was enhanced by supplying pH2 at a higher pressure which increased the pH2 available. While MRI
definitely can be improved and it is expected that HP can be further enhanced
along with dissolved pH2
and by optimization of the reaction (temperature, ccat) the HP levels were sufficient to demonstrate the in vivo applicability of SAMBADENA with
a 13C-angiography of an adult mouse. The results show the potential
of SAMBADENA to be a fast, simple and cost-efficient alternative method for the
production and application of HP 13C-tracers. The HP of biomolecules
like succinate11,12, lactate13–15, pyruvate or acetate16 is currently investigated.
Conclusion
In-vivo 13C-MRI of agents hyperpolarized by SAMBADENA without a dedicated polarizer was successfully demonstrated. With respect to recent advances in pH2-based methods concerning metabolic tracers, SAMBADENA appears well placed to simplify the research on - and application of - HP metabolic tracers. The next crucial step will be to adapt SAMBADENA to these new methods and metabolically active molecules.Acknowledgements
Support by the DFG (HO 4604/1-1 and HO 4604/2-1), the DKTK and the Heinrich-Böll-Stiftung (ABS, P131623) and the contribution of the COST Action TD1103 is greatly acknowledged. All animals were measured under the approval of the local ethics committee.References
1. Ardenkjaer-Larsen, J. H. et al. Dynamic nuclear polarization polarizer for sterile use intent. NMR Biomed. 24, 927–932 (2011).
2. Nelson, S. J. et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci. Transl. Med. 5, 108 (2013).
3. Cunningham, C. H. et al. Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circ. Res. CIRCRESAHA.116.309769 (2016). doi:10.1161/CIRCRESAHA.116.309769
4. Bowers, C. R. & Weitekamp, D. P. Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J. Am. Chem. Soc. 109, 5541–5542 (1987).
5. Schmidt, A. B. et al. Liquid-state carbon-13 hyperpolarization generated in an MRI system for fast imaging. Nat. Commun. (2017). doi:10.1038/ncomms14535
6. Berner, S. et al. Hyperpolarization of a biomolecule to 7% in an MRI using SAMBADENA. in EMIM 2017 (2017).
7. Hoevener, J.-B. et al. A continuous-flow, high-throughput, high-pressure parahydrogen converter for hyperpolarization in a clinical setting. NMR Biomed. 26, 124–131 (2013).
8. Haake, M., Natterer, J. & Bargon, J. Efficient NMR Pulse Sequences to Transfer the Parahydrogen-Induced Polarization to Hetero Nuclei. J Am Chem Soc 118, 88–91 (1996).
9. Bär, S. et al. On the spin order transfer from parahydrogen to another nucleus. J. Magn. Reson. 225, 25–35 (2012).
10. Gong, Q., Gordji-Nejad, A., Blümich, B. & Appelt, S. Trace Analysis by Low-Field NMR: Breaking the Sensitivity Limit. Anal. Chem. 82, 7078–7082 (2010).
11. Chekmenev, E. Y. et al. PASADENA Hyperpolarization of Succinic Acid for MRI and NMR Spectroscopy. J. Am. Chem. Soc. 130, 4212–4213 (2008).
12. Coffey, A. M. et al. Open-Source Automated Parahydrogen Hyperpolarizer for Molecular Imaging Using 13C Metabolic Contrast Agents. Anal. Chem. 88, 8279–8288 (2016).
13. Shchepin, R. V., Coffey, A. M., Waddell, K. W. & Chekmenev, E. Y. PASADENA Hyperpolarized 13C Phospholactate. J. Am. Chem. Soc. 134, 3957–3960 (2012).
14. Shchepin, R. V., Pham, W. & Chekmenev, E. Y. Dephosphorylation and biodistribution of 1-13C-phospholactate in vivo. J. Label. Compd. Radiopharm. 57, 517–524 (2014).
15. Cavallari, E., Carrera, C., Aime, S. & Reineri, F. 13C MR Hyperpolarization of Lactate by Using ParaHydrogen and Metabolic Transformation in Vitro. Chem. - Eur. J. 23, 1200–1204 (2017).
16. Reineri, F., Boi, T. & Aime, S. ParaHydrogen Induced Polarization of 13C carboxylate resonance in acetate and pyruvate. Nat. Commun. 6, 5858 (2015).
Figures