3571
A robust approach to generate high polarization levels of metabolites within seconds with para-hydrogen1NMR Signal Enhancement, Max-Planck-Institute for Biophysical Chemistry, Göttingen, Germany
Synopsis
Hyperpolarization of metabolites is a promising approach for in vivo disease detection and observation of treatment responses.1-4 Among hyperpolarization techniques, para-hydrogen induced polarization (PHIP) represents an inexpensive approach to generate polarization within a few seconds.5-11 Here, we are introducing a pulsed magnetic resonance method to polarize metabolites that enables us to efficiently transfer proton polarization to a 13C nucleus of interest. This becomes especially possible by attaching an optimized molecular sidearm to a metabolite of choice (here: acetate, glycine and pyruvate) which is para-hydrogenated and the polarization subsequently transferred. We have achieved high levels of metabolite precursor polarization (P >10%) with para-hydrogen within 15 seconds. Cleavage of the sidearm yields hyperpolarized metabolites.
Synopsis
Hyperpolarization of metabolites is a promising approach for in vivo disease detection and observation of treatment responses.1-4 Among hyperpolarization techniques, para-hydrogen induced polarization (PHIP) represents an inexpensive approach to generate polarization within a few seconds.5-11 Here, we are introducing a pulsed magnetic resonance method to polarize metabolites that enables us to efficiently transfer proton polarization to a 13C nucleus of interest. This becomes especially possible by attaching an optimized molecular sidearm to a metabolite of choice (here: acetate, glycine and pyruvate) which is para-hydrogenated and the polarization subsequently transferred. We have achieved high levels of metabolite precursor polarization (P >10%) with para-hydrogen within 15 seconds. Cleavage of the sidearm yields hyperpolarized metabolites.Purpose
To develop an inexpensive and fast approach (seconds) to deliver highly polarized metabolites for metabolic imaging in vivo.Methods
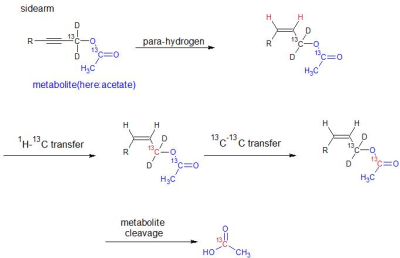
We have developed a molecular sidearm containing an unsaturated bond that can be linked to a metabolite of choice. This follows the recently introduced approach of PHIP via means of sidearm hydrogenation (PHIP-SAH).11 The sidearm contains a 13C species and the metabolite another 13C nuclei of interest. After the hydrogenation with para-hydrogen (90% para-enriched), the proton polarization is transferred to the first 13C nuclei in the sidearm and afterwards via a 13C-13C transfer to the 13C nuclei of interest (Figure 1). This polarization transfer is achieved with a newly designed pulse sequence. Afterwards the sidearm is cleaved with a base to yield the hyperpolarized metabolite. Metabolites that were attached to the sidearm include glycine, acetate and pyruvate.Results and Discussion
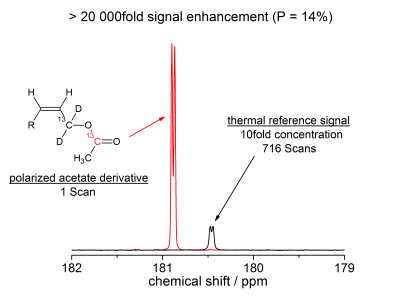
We have achieved signal enhancement levels of over 20 000fold (B0 = 7T) which corresponds to polarization levels of over 10%. The experimental time to generate these high levels of polarization is 15 seconds and an example of the hyperpolarized signal of the acetate precursor is displayed in figure 2. Experimentally, we verify over 40% transfer efficiency. During 10 seconds of reacting para-hydrogen with e.g. the acetate precursor, we generate 32% proton spin order. 22% Polarization was transferred to the first 13C nuclei and 14% to the 13C of acetate (figure 2). We would like to note that currently experiments are performed with an inexpensive bubbling setup and that the bubbling time is about 1.5 times the longitudinal proton relaxation time. If a more sophisticated setup, as presented in the literature,7-10 is being used for the hydrogenation step, the time is thought to be reduced to 1-3 seconds and we expect an immediate increase of polarization by a factor of two (P = 28%). The achieved levels of polarization are of relevance for in vivo experiments as similar levels are generated with dissolution dynamic nuclear polarization (DNP) devices before dissolution.2Conclusions
We have demonstrated that high levels of metabolite polarization with in vivo relevance can be generated within seconds by PHIP. This is achieved in two steps: (1) by optimizing a molecular sidearm system attached to a metabolite of interest that can be para-hydrogenated and (2) by utilizing a new pulse sequence allowing for an efficient polarization transfer to the 13C nuclei of a metabolite.Acknowledgements
The authors gratefully acknowledge funding from the Max-Planck-Society.References
1. Golman K, in ‘t Zandt R, Thaning M. Real-time metabolic imaging. Proc. Natl. Acad. Sci. USA 2006; 103:11270-11275.
2. Nelson SJ, Kurhanewicz J, Vigneron DB, et al. Metabolic Imaging of Patients withProstate Cancer
Using Hyperpolarized [1-13C] Pyruvate. Sci. Transl. Med. 2013; 198:198ra108.
3. Kurhanewicz J, Vigneron DB, Brindle K, et al. Analysis of Cancer Metabolism by Imaging
Hyperpolarized Nuclei: Prospects for Translation to Clinical Research. Neoplasia. 2011; 13:81-97.
4. Park I, Bok R, Ozawa T. et al. Detection of early response to temozolomide treatment in brain tumors using hyperpolarized 13C MR metabolic imaging. J. Magn. Reson. Imaging. 2011; 33:1284-1290.
5. Bowers CR, Weitekamp DP. Parahydrogen and Synthesis Allow Dramatically Enhanced Nuclear
Alignment. J. Am. Chem. Soc. 1987; 109:5541-5542.
6. Eisenschmid TC, Kirss RU, Deutsch PP, et al. Para Hydrogen Induced Polarization in Hydrogenation
Reactions. J. Am. Chem. Soc. 1987; 109:8089-8091.
7. Goldman M, Johannesson H, Axelsson O, Karlsson M. Design and implementation of 13C
hyperpolarization from para-hydrogen, for new MRI contrast agents. C. R. Chimie 2006; 9, 357-363.
8. Bhattacharya P, Chekmenev EY, Perman WH, et al. Towards hyperpolarized 13C-succinate imaging of brain cancer. J. Magn. Reson. 2007; 186:150-155.
9. Bhattacharya P, Chekmenev EY, Reynolds WF, et al. Parahydrogen-induced polarization (PHIP) b
hyperpolarized MR receptor imaging in vivo: a pilot study of 13C imaging of atheroma in mice. NMR Biomed. 2011; 24:1023-1028.
10. Schmidt AB, Berner S, Schimpf W, et al. Liquid-state carbon-13 hyperpolarization generated in an MRI system for fast imaging., Nat. Commun. 2017; 8:14535.
11. Reineri F, Boi T, Aime S. ParaHydrogen Induced Polarization of 13C carboxylate resonance in acetate and pyruvate. Nat. Commun. 2015; 6:5858