3570
Improved Hyperpolarized Cerebral Perfusion Imaging Using a Sucrose/Water Glassing Matrix for tert-Butanol1Division of MR Research, Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States
Synopsis
Perfusion imaging is a promising application for hyperpolarized tracers, as they provide high signal with no endogenous background. Hyperpolarized 13C labeled tert-butanol is a freely diffusible perfusion agent with long T1 and T2 relaxation times in vivo. Prior work has shown that tert-butanol can be polarized to 5-10% using dynamic nuclear polarization through addition of glycerol as a glassing agent. Here we investigate a formulation based on a water/sucrose/tert-butanol mixture that yields a 1.6-fold improvement in polarization, and illustrate its use in 3D cerebral perfusion imaging in rats.
Purpose:
To develop improved hyperpolarized cerebral perfusion imaging by making use of a new glassing matrix for dynamic nuclear polarization of 13C labeled tert-butanol.Introduction:
Hyperpolarized tert-butanol provides high quality perfusion weighted images in the brain and body owing to its long T1 and T2 relaxation times and its ability to diffuse through biological membranes, including the blood-brain barrier [1]. Hyperpolarization of tert-butanol using dynamic nuclear polarization (DNP) requires preparation of solutions that form glassy solids when frozen. Previously, a glycerol/tert-butanol mixture together with FINLAND radical was shown to yield 5-10% polarization levels at 1.4K and 3.35T. Here we investigate an alternative formulation based on a water/sucrose/tert-butanol mixture and OX063 radical. We also investigate the effects of adding gadolinium and holmium chelates to the preparations [2].Methods:
All data were acquired using a 9.4T scanner (Biospec 94/20, Bruker, Billerica MA) equipped with a 28mm transmit/receive 13C surface coil (for in vivo imaging) or a 36mm 13C saddle coil (for polarization measurements).
Agent preparation: FINLAND and OX063 were obtained from Oxford Instruments (Oxfordshire UK) and DOTA was obtained from Macrocyclics (Plano, TX). All other reagents were obtained from Sigma (St Louis, MO). The FINLAND/glycerol formulation was prepared by combining glycerol and tert-butanol (50/50 v/v) with 16mM FINLAND. The OX063/sucrose formulation was prepared by combining tert-butanol with water (50/50 v/v) and then adding 32mg of sucrose per 100mg of tert-butanol/water mixture. 16mM OX063 was then added. Aliquots of each formulation were prepared containing 0,1,2 or 4mM of either gadoteridol (Bracco Diagnostics) or holmium-DOTA. For in vivo imaging, 1mM gadoteridol was added to the FINLAND/glycerol formulation, and 1.4mM holmium DOTA was added to the sucrose/water formulation.
Polarization and T1 Measurement: 75mg samples were polarized as described previously [1]. Following dissolution, solutions were transferred to a syringe and injected through a thin tube into a vial situated within the scanner. A single spectrum was then acquired with a 45 degree pulse approximately 15s after the end of the dissolution. This was followed by a train of 3 degree pulses (TR=5s) for T1 measurement. The thermal signal was then measured with 45 degree tip angle, TR=240s, and 6 or more signal averages to enable computation of the initial polarization.
In Vivo Imaging: Two female Sprague-Dawley rats were imaged with IACUC approval. Rats were prepared for imaging as described previously [1]. After acquisition of proton anatomic images, a bolus of hyperpolarized solution was injected, and 13C image acquisition was initiated at the end of the injection using a 3D balanced steady-state free precession sequence (TR/TE=2.3/1.15ms, 25 degree tip angle). In a first experiment the animal received two 2ml injections, separated by approximately 90 minutes, prepared with FINLAND/glycerol and OX063/sucrose mixtures, respectively. After each injection, sixty-four 3D frames were acquired with 40x26x20 matrix and 1.2mm isotropic resolution. In a second animal, 270mg of the OX063/sucrose formulation were polarized, 3ml of the hyperpolarized solution were injected, and twenty-four 3D frames were acquired with 40x40x32 matrix and 0.7mm isotropic resolution.
Image Reconstruction: k-space data were weighted and summed as described previously [1], zero-filled to 4 times the acquired matrix size, and Fourier transformed. Complex images were multiplied by a constant overall phase to obtain a real-valued signal intensity, and the real part of the images were used for display and analysis.
Results and Discussion:
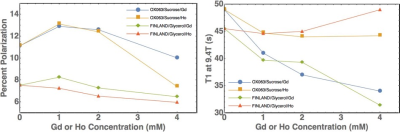
Figure 1 shows polarization levels and T1 relaxation times as a function of holmium and gadolinium concentration for the two preparations. Holmium and gadolinium provide little or no benefit in the FINLAND/glycerol formulation, with the peak signal obtained with ~1mM gadolinium. The OX063/sucrose preparation shows roughly 1.6-fold improvement in polarization, with gadolinium and holmium yielding comparable improvements in polarization. The holmium preparations have a longer liquid-state T1 than those containing gadolinium, as expected [2].
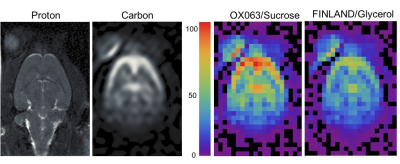
Figure 2 shows a proton reference image and a representative perfusion-weighted image acquired in the animal that received two bolus injections. The right panels show a comparison of SNR between the FINLAND/glycerol and OX063/sucrose formulations. For the slice shown, the OX063/sucrose preparation yields a peak SNR of 107, a roughly 1.5-fold improvement over FINLAND/glycerol, after correcting for the 3.5% lower t-butanol dose in the FINLAND/glycerol scan.
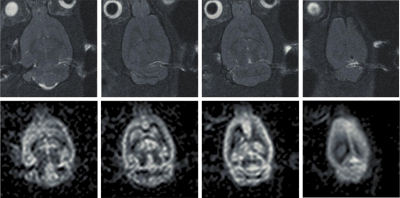
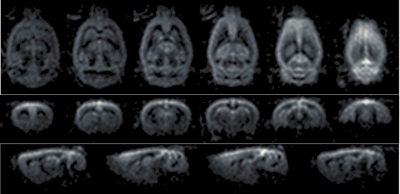
Figure 3 displays representative proton and perfusion-weighted images acquired in the second rat at 700 micron isotropic resolution. Figure 4 shows reformatted axial, coronal and sagittal images from this data set.
Conclusions:
The sucrose/water glassing matrix presented here provides improved polarization and in vivo SNR. These improvements enable cerebral perfusion imaging in rats with 700µm isotropic resolution. These techniques may also provide a strategy for co-polarization of t-butanol with other sugars including fructose and glucose [3,4].Acknowledgements
This work was supported in part by the National Institutes of Health through grants R21 EB014471 and R01 CA169470.References
[1] Grant AK, Vinogradov E, Lenkinski RE, Alsop DC. Perfusion Imaging with a Freely Diffusible Hyperpolarized Contrast Agent. Magn Reson Med 66:746-755 (2011).
[2] Kiswandhi A, Niedbalski P, Parish C, Kaur P, Martins A, Fidelino L, Khemtong C, Song L, Sherry AD and Lumata L. Impact of Ho3+-doping on 13C dynamic nuclear polarization using trityl OX063 free radical. Phys. Chem. Chem. Phys. 18:21351 (2016).
[3] Keshari KR, Wilson DM, Chen AP, Bok R, Larson PEZ, Hu S, Van Criekinge M, Macdonald JM, Vigneron DB, and Kurhanewicz J. Hyperpolarized [2-13C] fructose: a hemiketal DNP substrate for in vivo metabolic imaging. J Am Chem Soc 131:17591–17596 (2009).
[4] Rogrigues TB, Serrao EM, Kennedy BWC, Hu D, Kettunen MI, Brindle KM. Magnetic resonance imaging of tumor glycolysis using hyperpolarized 13C-labeled glucose. Nature Medicine 20:93-98 (2014).
Figures