3566
Probing renal pH and aminopeptidase N activity with hyperpolarized [1-13C]alaninamideAlice Radaelli1, Hikari Ananda Infinity Yoshihara1, Ryunosuke Hata2, Shinsuke Sando3, and Rolf Gruetter1,4
1Laboratory for Functional and Metabolic Imaging (LIFMET), EPFL, Lausanne, Switzerland, 2Department of Chemistry and Biochemistry, Kyushu University, Fukuoka, Japan, 3Department of Chemistry and Biotechnology, The University of Tokyo, Tokyo, Japan, 4Center for Biomedical Imaging (CIBM), Lausanne, Switzerland
Synopsis
The detection of aminopeptidase N (APN) activity can give information on tumor development. Hyperpolarized L-[1-13C]alaninamide is a specific, sensitive probe of APN activity in kidney homogenate. Here, we characterized its in vivo metabolic response in the rat kidney. In addition to being an APN substrate, L-[1-13C]alaninamide is sensitive to pH and also reacts with dissolved carbon dioxide. To avoid spectral overlap, alaninamide is best suited as an APN probe in acidic environments, and it may have additional applications as a multifunctional sensor of pH and CO2.
Introduction
Aminopeptidase N (APN) is an enzyme that removes the N-terminal amino acid residue from peptides or proteins. It plays different physiological roles, and it has been recognized as a tumor biomarker1 as it is involved in tumor angiogenesis. Because of the challenges presented by the detection of APN activity with fluorescent or luminescent probes in opaque biological samples, NMR probes are good candidates to monitor its enzymatic activity. Hata et al.2, have recently demonstrated the specificity of alaninamide for APN activity in kidney homogenate. The present study aims to explore the possibility of using hyperpolarized L-[1-13C]alaninamide to detect APN activity in vivo in the rat kidney.Methods
Samples of 4.4 M of L-[1-13C]alaninamide∙HCl doped with 25 mM of OX063 radical were polarized in a custom-built 7 T polarizer at 1 K by shining microwave radiation at 196.59 GHz, 50 mW in power. The polarization buildup was monitored for approximately 2 hours, after which the samples were rapidly dissolved in 5.5 mL of a hot buffer solution (pH = 7.4) and transferred (3 s) to a separator infusion pump located in a 9.4 T/ 31 cm animal scanner. n = 4 male Sprague Dawley rats were anaesthetized using 1-2% isoflurane. 1.2 mL of the hyperpolarized alaninamide solution was injected through the femoral vein over 9 s. The bolus concentration prior to infusion was ≈ 6.4 mM. The 13C signal acquisition was performed with respiration and cardiac gating. 1H-decoupled 13C FIDs were acquired by applying 30° BIR4 pulses using a single loop 1H / quadrature 13C surface coil placed over the left kidney of the animal. The repetition time between each scan was ≈ 3 s. Blood gases, pH and physiological parameters were monitored shortly after each infusion. For each infusion, the acquired FIDs were fitted with Bayes (Washington University, St. Louis) to determine the spectral peak amplitudes and the ratios between metabolites. The alaninamide titration curve was determined by performing 13C NMR measurements at 9.4 T, 37° on a set of 1 M Ala-NH2∙HCl samples with pH ranging between 3.55 and 10.16, referenced to 15 mM 13C urea. Carbamate formation was monitored via 13C NMR (9.4 T, 37°) in a pH 8 solution of 10 mM L-[1-13C]alaninamide ∙HCl, 100 mM NaH13CO3, and 5 mM 13C urea.Results and discussion
The most striking feature of the 13C spectrum of alaninamide is the presence of a three-fold split alaninamide peak (Fig. 1), which arises from the 1-13C sensitivity to protonation and deprotonation of the amine. The alaninamide acid-base titration curve (Fig. 2) shows a 13C chemical shift change of ≈ 8.4 ppm and pKa of 7.9. The three peaks correspond to three different extracellular pH compartments within the kidney: pH = 7.46 ± 0.05, pH = 7.22 ± 0.09 and pH 6.58 ± 0.05. Based on literature3,4, the three compartments can be tentatively assigned to the cortex/blood, medulla and calyx/ureter. Because of the overlap in chemical shift with the pH = 7.46 compartment peak, the resolution of alanine is limited and strongly depends on the pH value. A second metabolic product appears from the very first scan at 183.67 ppm: the metabolite was identified as carbamate forming from the reaction of alaninamide with CO2 present in the blood (Fig. 3).Conclusions
The in vivo renal metabolism of hyperpolarized L-[1-13C]alaninamide was studied. The probe was observed to be extremely sensitive to pH variations within the kidney. Alanine production was observed, as well as carbamate formation from the reaction of the parent compound with CO2. Although the poor resolution of the alanine peak limits its use as APN probe in the kidney, lower-pH tissues may be better for this application.Acknowledgements
This work was supported by the Swiss State Secretariat for Education, Research and Innovation (SERI) within the Marie Curie Initial Training Network EUROPOL project (n° SERI: 15.0164), and by the Centre d'Imagerie Biomédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, and the Leenards and Jeantet Foundations.References
1. Wickström et al., Cancer Sci. 102 (2011)
2. Hata et al., Angew. Chem. Int. Ed. 55 (2016)
3. Duewel et al., Nat. Commun. 8 (2017)
4. Raghynand et al.,MRM 49 (2003)
Figures
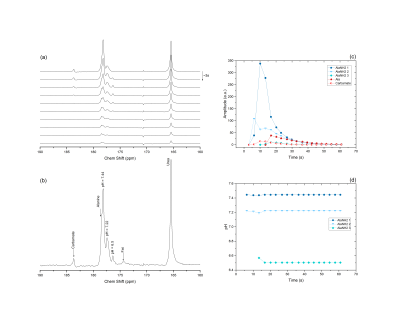
Figure 1: (a)
Representative dynamic spectra acquired in the rat left kidney after a bolus
injection of L-[1-13C]alaninamide. 10 of the acquired spectra are displayed.
(b) Sum of the acquired spectra. (c) Timecourse of the spectral data. (d) pH
variation as a function of time of the three alaninamide compartments.
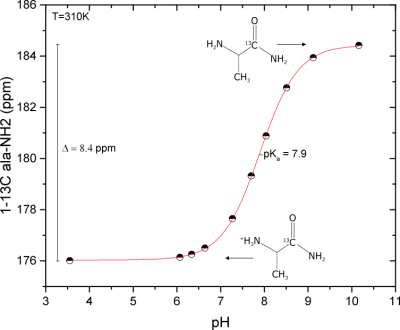
Figure 2:
L-[1-13C]alaninamide 13C NMR acid-base titration curve measured at
37°.
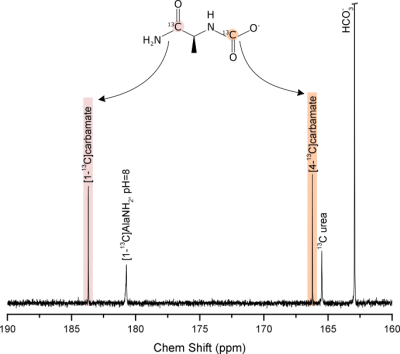
Figure 3: 13C NMR spectrum acquired at
37° of a pH=8 solution of 10 mM L-[1-13C]alaninamide, 100 mM NaH13CO3
and 5 mM 13C urea. Carbamate formation (C1 at 183.68 ppm,
C4 at 166.21 ppm) is observed.