3563
Mitigating Chemical Exchange Effects in Advanced Pulse Sequences for pH Imaging with Hyperpolarized [13C]bicarbonate1Radiology and Biomedical Imaging, UC San Francisco, San Francisco, CA, United States, 2Bioengineering, UC San Francisco, San Francisco, CA, United States
Synopsis
Imaging extracellular acidification in tumors will likely lead to better characterization of tumor aggressiveness and treatment efficacy. Hyperpolarized (HP) [13C]bicarbonate magnetic resonance spectroscopic imaging (MRSI) can map pH in murine tumors, but images generally suffer from low signal-to-noise ratio (SNR) and coarse spatial resolution. Although sophisticated pulse sequences can boost SNR, pH accuracy can be compromised due to bidirectional [13C]bicarbonate <-> 13CO2 chemical exchange during imaging. We investigated several pulse sequences and excitation/refocusing schemes, and a modified 2D echo-planar imaging sequence with spectral-spatial excitation demonstrated the best combination of spatial resolution, pH accuracy, and potential for future clinical implementation.
Purpose
To investigate and mitigate detrimental effects of rapid chemical exchange on imaging signal-to-noise ratio (SNR) and pH measurement accuracy for HP pH imaging with [13C]bicarbonateMethods
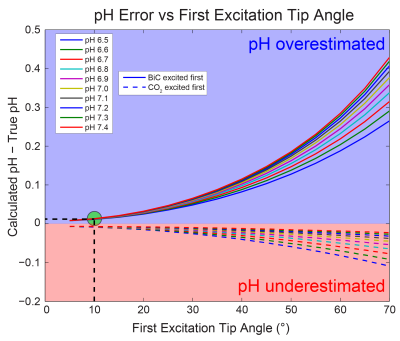
Bicarbonate-CO2 exchange modeling: The imaging pH error was simulated as a function of true pH, tip angle on the first excited resonance, and order of excitation (bicarbonate first or CO2 first). Bicarbonate and CO2 z-magnetizations were first initialized based upon the true pH value. The first resonance was then sampled, and the remaining total z-magnetization was distributed between the two pools assuming complete chemical exchange. The new metabolite pools were then adjusted for T1 relaxation (assuming the same T1 for both metabolites1,2), and the second metabolite was then sampled with a 90° pulse. The ratio of the acquired signals was tip angle-corrected and used to calculate the measured pH with a modified Henderson-Hasselbalch equation (pKa = 6.17)1.
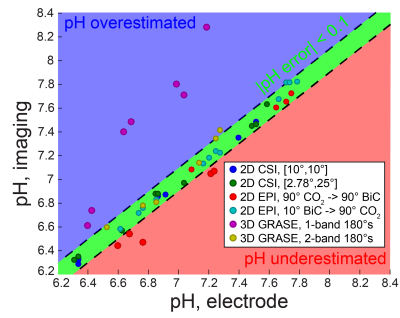
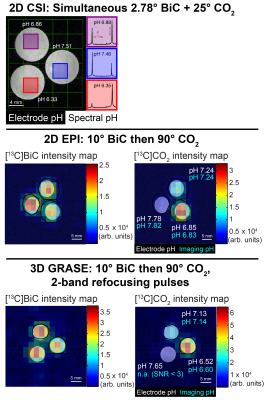
Phantom pH imaging: 13C magnetic resonance spectroscopic imaging (MRSI) was performed at 37 °C on three tubes containing HP [13C]bicarbonate generated via HP [1-13C]1,2-glycerol carbonate decarboxylation3 in 100 mM phosphate, pH 6.4-7.6, and 7.5 µg/mL carbonic anhydrase II in a vertical-bore 14 T scanner. Three imaging sequences were employed: 2D chemical shift imaging (CSI), 2D echo-planar imaging (EPI), and 3D gradient spin-echo (GRASE)4 imaging. The resonance-specific tip angles (bicarbonate:CO2 = 1:1 or 1:9), metabolite excitation order, and 180° pulse selectivity (one or both resonances) were varied for each pulse sequence. The pH values were measured afterward at 37 °C by pH electrode.
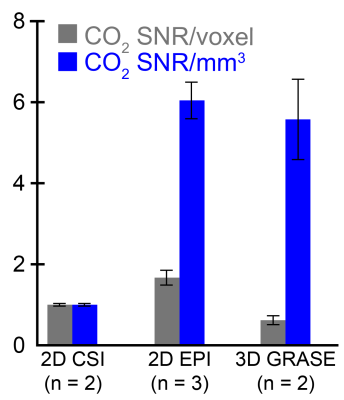
Image processing: All 13C imaging data were processed using custom MATLAB scripts except 2D CSI spectral data (processed using SIVIC open-source software). 13C images were spatially zero-filled and/or apodized (spectrally), pH maps were calculated from bicarbonate and CO2 using tip angle correction and a modified Henderson-Hasselbalch equation (pKa = 6.17)1, mean pH values were computed from a region of interest (ROI) drawn within each tube, and pH error was calculated relative to electrode measurements. Because low CO2 SNR typically limits imaging spatial resolution, CO2 SNR was compared between imaging sequences by averaging the CO2 SNR over all voxels and normalizing by the CO2 SNR acquired from a 1° slab pulse-acquire immediately before imaging.
Results
Modeling of the bicarbonate-CO2 exchange, assuming complete exchange following excitation, led to the identification of 10° bicarbonate followed by 90° CO2 as an excitation scheme that keeps |pH error| < 0.015 unit (Figure 1). The accuracy of pH measurement via imaging was evaluated using this excitation scheme, along with other non-ideal schemes (Figure 2). Several imaging parameters, including tip angles applied, order of excitation, and refocusing one or both resonances, were varied. Generally, pH accuracy < 0.1 pH unit was lost if the CO2 was excited first with a high tip angle (eg. 90°), or if the sequence employed a low initial tip angle on bicarbonate followed by selective bicarbonate refocusing. This was accompanied by a decrease in SNR of the second excited resonance, particularly for the 3D GRASE sequence (data not shown). Figure 3 displays representative HP images obtained with 2D CSI, 2D EPI, and 3D GRASE pulse sequences under optimal imaging parameters. Between these sequences, the SNR of the CO2 resonance was highest on a per-volume basis for both the 2D EPI and 3D GRASE sequences (Figure 4).Discussion
Because in vivo bicarbonate-CO2 exchange can be rapid relative to the timescale of HP imaging, care must be taken in utilizing HP magnetization. If bicarbonate and CO2 are excited sequentially, the first resonance must be excited minimally in order to reduce perturbations to the total HP pool and preserve pH accuracy. Furthermore, refocusing schemes must invert both resonances simultaneously; otherwise, chemical exchange of their out-of-phase z-magnetizations can cancel each other out and reduce the signal when imaging the second excited resonance. Based upon simulation of complete bicarbonate-CO2 exchange, we were able to obtain excitation parameters that demonstrated high-SNR pH imaging in phantoms at 14 T, especially compared with a typical 2D CSI approach.Conclusions
We have developed a 2D EPI sequence with spectral-spatial excitation that provided high spatial resolution pH imaging with high accuracy. This type of HP imaging sequence is readily applied to clinical imaging and has potential for future implementation for patient tumor pH measurement with HP [13C]bicarbonate.Acknowledgements
The authors wish to thank all members of the Flavell and Kurhanewicz Labs.
Grants: R01-CA166655; R01-EB016741; P41-EB013598; DOD-CA-110032.
References
1. Gallagher, F. A. et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 453, 940–943 (2008).
2. Wilson, D. M. et al. Multi-compound polarization by DNP allows simultaneous assessment of multiple enzymatic activities in vivo. J Magn Reson 205, 141–147 (2010).
3. Korenchan, D. E. et al. Dynamic nuclear polarization of biocompatible 13C-enriched carbonates for in vivo pH imaging. Chem Commun 52, 3030–3033 (2016).
4. Oshio, K. & Feinberg, D. A. Single-shot GRASE imaging without fast gradients. Magn Reson Med 26, 355–360 (1992).
Figures