3559
Optimized Single Shot 3D Sequence for High Resolution Hyperpolarized 13C Imaging1Cancer Research UK Cambridge Institute, University of Cambridge, Cambridge, United Kingdom, 2Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom
Synopsis
We have developed a single-shot 3D sequence for hyperpolarized 13C MRI, which uses a spatial-spectral (SpSp) pulse for excitation, a train of adiabatic pulses for refocusing, and a stack-of-spirals acquired through a fast-spin-echo train. The sequence achieved an isotropic image resolution of 1.25x1.25x1.25 mm3 in vivo on a 7 T animal system, where hyperpolarized [1-13C]pyruvate and [1-13C]lactate were imaged alternately at a frame rate of 2 s per metabolite. Signals from extra spirals acquired from later spin echoes were averaged with those from the early echoes to give a high signal-to-noise ratio (SNR) as well as high spatial resolution.
Introduction
Hyperpolarized 13C-labeled metabolites have enabled real time metabolic imaging in vivo [1]. The transient hyperpolarized signal and heterogeneous nature of tumors require rapid acquisition of high spatial resolution images. Excitations with RF pulses should be limited as each excitation depletes some of the hyperpolarization. A single-shot 3D sequence was developed to match these demands, which achieved a spatial resolution of 1.25x1.25x2.5 mm3 in vivo at a rate of up to 200 ms/image on a 7 T animal scanner [2]. Here we present an improved sequence that doubled the spatial resolution at essentially no cost in imaging speed and minimal cost in terms of SNR.Methods
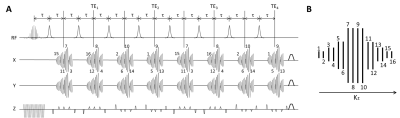
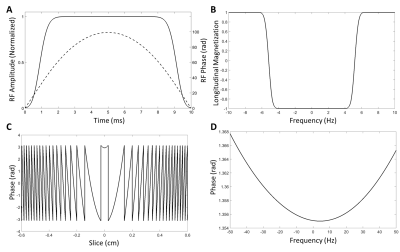
Following each SpSp excitation pulse, the pulse sequence acquires eight stacks of four spiral acquisitions such that each kxy plane in 3D k-space is encoded by a spiral-out readout (Figure 1). A train of blipped gradients along the z-axis encode spatial information in the third dimension. Signals from the last four stacks were averaged with signals from the first four to increase the SNR. Each stack of spirals was acquired at a spin echo formed by an unpaired adiabatic inversion pulse (Hyperbolic-Secant). The inversion pulses were used without slice-selection gradients, such that the non-linear phase imparted by the pulses was kept to a minimum and depended solely on B0 homogeneity. A comparison of the non-linear phase produced with and without a slice-selection gradient is shown in Figure 2 for the adiabatic pulse used in the sequence.
Experiments were performed on a 7 T scanner with 3 female C57BL/6J mice bearing EL4 lymphoma tumors and in compliance with a project license issued under the Animals (Scientific Procedures) Act of 1986. Protocols were approved by a local Animal Welfare and Ethical Review Body. The [1-13C]pyruvate sample, containing 44 mg [1-13C]pyruvate acid (CIL, MA, USA), 15 mM OXØ63 (GE Healthcare, Amersham, UK) and 1.4 mM gadoterate meglumine (Dotarem; Guerbet, Roissy, France), was hyperpolarized using a Hypersense Polarizer (Oxford Instruments, Abingdon, UK) at 1.2 K in a magnetic field of 3.35 T and dissolved in 6 ml buffer with 40 mM Tris, 185 mM NaOH, and 100 mg/L EDTA heated to 180 °C and pressurized at 10 bar. Imaging started 2 seconds after injection of 400 µl of ~ 80 mM hyperpolarized [1-13C]pyruvate solution, with a FOV of 4x4x2 cm3 and a nominal resolution of 1.25x1.25x1.25mm3. As indicated in Figure 1, the spirals at the center of k-space in the kz direction encode matrices with a nominal size of 32x32. The encoded matrix size decreases to 16x16, 8x8, and 4x4 as the spirals move to the periphery in the kz direction. The spiral lengths were 8.500, 3.120, 1.216, and 0.552 ms respectively. The 10.016 ms SpSp pulse had an excitation bandwidth of 350 Hz and the center-center distance between two replicate bands was 1645 Hz. Five [1-13C]pyruvate images were acquired at a 2 s frame rate before the first [1-13C]lactate image was acquired, 1 s after the 5th [1-13C]pyruvate image. [1-13C]pyruvate and [1-13C]lactate images were acquired in alternate order thereafter, hence there was a temporal resolution of 2 s/frame for each metabolite. The flip angles on [1-13C]pyruvate and [1-13C]lactate were 7° and 45° respectively.
Results
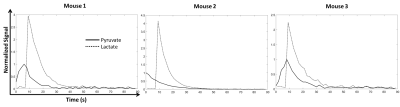
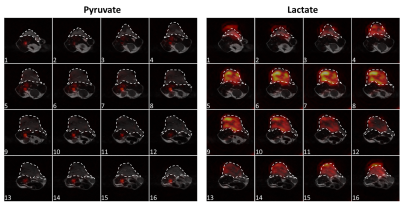
Time courses of the [1-13C]pyruvate and [1-13C]lactate signals acquired following i.v. injection of hyperpolarized [1-13C]pyruvate in tumor-bearing mice are shown in Figure 3. The time courses are the summation of signals from all the voxels in the central 10 slices at each time point. Representative [1-13C]pyruvate and [1-13C]lactate images are shown in Figure 4, where considerable heterogeneity could be observed in both the xy plane and the z direction.Discussion and Conclusion
The pulse sequence doubles the spatial resolution when compared to a single shot 3D imaging technique described previously [2]. The decreased SNR resulting from the increased spatial resolution is partially recovered by acquiring more echoes, exploiting the long T2 of 13C-labeled metabolites. The isotropic and high image resolution not only can reveal more heterogeneity in tumor metabolism but also potentially enhance co-registration with 18F-PET images, which can be sub-millimeter resolution and are naturally isotropic [3].Acknowledgements
The work was supported by a Cancer Research UK Programme grant (17242) to KMB and by the CRUK-EPSRC Imaging Centre in Cambridge and Manchester (16465). JW was also supported, in part, by a grant from the Danish Strategic Research Council (LIFE-DNP: Hyperpolarized magnetic resonance for in vivo quantification of lipid, sugar and amino acid metabolism in lifestyle related diseases).References
[1] Brindle KM. Imaging metabolism with hyperpolarized 13C labeled cell substrates. J Am Chem Soc 2015; 137:6418-6427.
[2] Wang J, Wright AJ, Hu DE, Hesketh R, Brindle KM. Single shot three-dimensional pulse sequence for hyperpolarized 13C MRI. Magn Reson Med 2017;77(2):740-752.
[3] Gutte H, Hansen AE, Henriksen ST, Johannesen HH, Ardenkjaer-Larsen J, Vignaud A, Hansen AE, Borresen B, Klausen TL, Wittekind AM, Gillings N, Kristensen AT, Clemmensen A, Hojgaard L, Kjaer A. Simultaneous hyperpolarized 13C-pyruvate MRI and 18F-FDG-PET in cancer (hyperPET): feasibility of a new imaging concept using a clinical PET/MRI scanner. Am J Nucl Med Mol Imaging 2015;5(1):38-45.
Figures