3557
The effect of ¹H-decoupling on hyperpolarized ¹³C signal decay – a study on choline chloride analogs and comparison to the effect of deuterium substitution of directly bonded protons1Hadassah-Hebrew University Medical Center, Jerusalem, Israel
Synopsis
The spin-lattice relaxation time (T1) of a DNP molecular probe is a key parameter in acquiring NMR signals in dissolution-DNP (d-DNP) experiments. Using molecular probes with long T1, NMR spin signals can survive for a duration sufficient for the study of metabolism (1-3 min). Deuteration of directly bonded 13C protonated positions has been useful in prolonging the visibility of hyperpolarized labeled 13C sites that are otherwise protonated. Here, we sought to investigate whether proton irradiation could affect the T1 of such 13C nuclei when such positions are in their naturally abundant form, i.e. directly bonded to protons.
Introduction
In dissolution-DNP (d-DNP) [1] experiments, the spin-lattice relaxation time (T1) of the DNP molecular probe (the substrate) defines the survival time of the NMR signal for the detection of that probe. For this reason, a major consideration in the selection of a DNP probe is its T1. The most useful 13C positions for metabolic research are non-protonated, like [1-13C]pyruvate. For protonated 13C positions, the presence of heteronuclear 13C-1H interactions and their cross-relaxation [2, 3] affects the T1 times of those 13C nuclei. Employing proton (1H) decoupling [4-9], a reduction in the T1 of 13C nuclei was reported using substrates at thermal equilibrium [10]. Using the pre-polarized [1-13C]lactate, it was later observed that there was no such effect of 1H decoupling on T1 of 13C nucleus and a marginal increment in T1 was reported [11]. Deuteration of proton positions either directly bonded or adjacent to 13C nuclei is a useful method to prolong the T1 of 13C nuclei at thermal equilibrium [12] and in a hyperpolarized state [13, 14]. Here we aimed at investigating the effects of proton decoupling on the T1 of 13C in positions that are directly bonded to two protons (methylene positions) in a hyperpolarized state, and comparing these possible effects to the known prolongation effect of deuterium substitution in the same molecule. This was possible using the choline analogs [1,2-13C]choline chloride and [1,1,2,2-D4,1-13C]choline chloride.Methods
The OXO63 radical (GE Healthcare, UK) was obtained from Oxford Instruments Molecular Biotools (Oxford, UK). [1-13C]pyruvic acid was purchased from Sigma-Aldrich (SA, Rehovot, Israel) and from Cambridge Isotope Laboratories (CIL, Tewksbury, MA, USA). 13C NMR spectra were acquired using a 5.8 T spectrometer (RS2D, Mundolsheim, France). Dissolution dynamic nuclear polarization (d-DNP) was performed using a spin polarizer (HyperSense, Oxford instruments, Oxford, UK). Dissolution was performed in 4 ml of D2O. Spectral processing and calculation of integrated intensities were performed using MNova (Mestrelab Research, Santiago de Compostela, Spain). T1 measurements of 13C nuclei in [1,2-13C]choline (Fig. 1a) and its deuterated analog, [1,1,2,2‐D4,1‐13C]choline (Fig. 1b) were conducted in a hyperpolarized state. 1H decoupling was performed with WALTZ-16 [15]. Due to the decay of hyperpolarized magnetization with cosθ, we acquired 13C signals with low flip angles in all the experiments. To determine the T1 of 13C nuclei, we employed the equation, M(t)=M0.e(-t/T1).cosθ(t/TR) where 'TR' refers to the time interval between the excitations, 'θ' is the RF flip angle and 't' is the time at the end of each acquisition. Curve fitting was performed using Matlab (Mathworks, Natick, MA, USA).Results
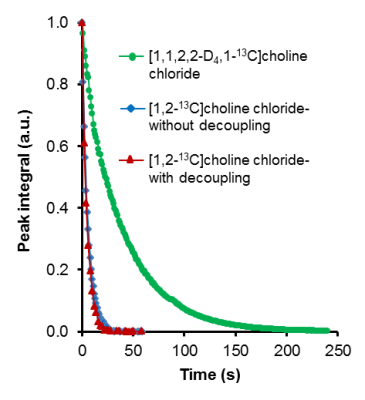
The decay of the hyperpolarized state of the 13C positions in choline analogs is depicted in Figure 2. In Fig. 2(a), [1,1,2,2-D4,1-13C]choline shows only the C1 signal and the C2-position is not labeled. Fig. 2(b) and 2(c) show both C1 and C2 signals without and with 1H decoupling, respectively. The signal decay of the C1 position in all three cases is depicted in Fig. 3. The T1 values of both C1 and C2 from choline as well as deuterated choline are summarized in Table 1.Discussion and Conclusions
Comparing the spilt pattern in Fig. 2(b) and 2(c), it can be seen that the 13C signals were resolved into doublets, i.e. only the 13C-13C coupling remained in the presence of 1H irradiation, as expected. T1 measurements suggested that there was a decrease in T1 times of both positions due to proton irradiation (Table 1). The decay curves shown in Fig. 3 depict this trend as well. Since this study used controlled conditions, i.e. the same molecules, the same dissolution conditions, and the same measurement approach, we can conclude that proton irradiation slightly reduces the apparent T1 decay of hyperpolarized protonated 13C positions. Although percentage wise the effect could appear large (20-25 %), the effect on the ability to detect the protonated signals in a hyperpolarized signal is small as all values are on the shorter end of T1 useful for hyperpolarized studies. In this sense, the deuterium substitution of directly bonded positions is a superior method to enable visualization of such molecules on d-DNP studies.Acknowledgements
This work was supported by the European Research Council (Grant No. 338040).References
1. Ardenkjær-Larsen, J.H., et al., Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proceedings of the National Academy of Sciences, 2003. 100(18): p. 10158-10163.
2. Levy, G.C., Carbon-13-spin-lattice relaxation studies and their application to organic chemical problems. Accounts of Chemical Research, 1973. 6(5): p. 161-169.
3. Campbell, I.D. and R. Freeman, Influence of cross-relaxation on NMR spin-lattice relaxation times. Journal of Magnetic Resonance, 1973. 11(2): p. 143-162.
4. Bloom, A.L. and J.N. Shoolery, Effects of Perturbing Radiofrequency Fields on Nuclear Spin Coupling. Physical Review, 1955. 97(5): p. 1261-1265.
5. Anderson, W.A. and R. Freeman, Influence of a Second Radiofrequency Field on High‐Resolution Nuclear Magnetic Resonance Spectra. The Journal of Chemical Physics, 1962. 37(1): p. 85-103.
6. Baldeschwieler, J.D. and E.W. Randall, Chemical Applications of Nuclear Magnetic Double Resonance. Chemical Reviews, 1963. 63(2): p. 81-110.
7. Ernst, R., Nuclear Magnetic Double Resonance with an Incoherent Radio‐Frequency Field. The Journal of Chemical Physics, 1966. 45(10): p. 3845-3861.
8. Mehring, M., Principles of High Resolution NMR in solids. (Springer, Berlin, 1983), 2nd edn.
9. Haeberlein, U., High resolution NMR in solids: selected averaging. 1976: Academic Press.
10. Day, I.J., et al., Applications of DNP-NMR for the measurement of heteronuclear T 1 relaxation times. Journal of Magnetic Resonance, 2007. 187(2): p. 216-224.
11. Chen, A.P., et al., In vivo hyperpolarized 13 C MR spectroscopic imaging with 1 H decoupling. Journal of Magnetic Resonance, 2009. 197(1): p. 100-106.
12. Akasaka, K., et al., Deuterium substitution effect on relaxation times (DESERT) and its application to the study of conformation of some purine nucleoside derivatives. Journal of Magnetic Resonance, 1975. 18(2): p. 328-343.
13. Allouche‐Arnon, H., et al., Deuteration of a molecular probe for DNP hyperpolarization–a new approach and validation for choline chloride. Contrast media & molecular imaging, 2011. 6(6): p. 499-506. 14. Allouche‐Arnon, H., et al., A hyperpolarized choline molecular probe for monitoring acetylcholine synthesis. Contrast media & molecular imaging, 2011. 6(3): p. 139-147.
15. Shaka, A., J. Keeler, and R. Freeman, Evaluation of a new broadband decoupling sequence: WALTZ. Journal of Magnetic Resonance, 1983. 53(2): p. 313-340.
Figures

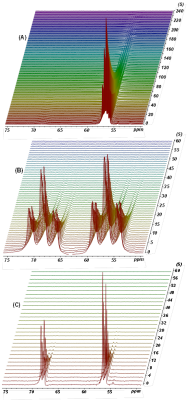
Figure 2. 13C NMR spectra obtained from dissolution-DNP experiments.
(A) The C1 signal of [1,1,2,2‐D4,1‐13C]choline chloride in D2O at TR=1 s. (B) The C1 and C2 signals of [1,2-13C]choline chloride in D2O without 1H decoupling at TR=1 s.
(C) The C1 and C2 signals of [1,2-13C]choline chloride in D2O with 1H decoupling (WALTZ-16) at TR=2 s.