3556
A perfused heart system to simulate first pass observation of rat cardiac metabolism with hyperpolarized [1-¹³C]pyruvate and determination of LDH flux using selective excitation1Radiology, Hadassah-Hebrew University Medical Center, Jerusalem, Israel
Synopsis
Aberrant cardiac metabolism is linked to major health issues in the Western world including diabetes and heart failure. New tools are needed to investigate these conditions and to allow better diagnosis. In this work we used dissolution dynamic nuclear polarization NMR spectroscopy (dDNP-NMR) to investigate [1-13C]pyruvate metabolism in the isolated rat heart. A perfusion system simulating in vivo first-pass hemodynamics was used to measure the enzymatic flux through lactate dehydrogenase (LDH) using product selective excitation. LDH flux was found to be 52 ± 8 nmol lactate/s/g wet weight (n=3).
Introduction
Heart failure and diabetes are leading causes of morbidity and mortality in the Western world. In recent years it was discovered that these conditions involve aberrant cardiac metabolism, driving the quest for new tools enabling metabolic research in real-time. The perfused rodent heart is an established system for monitoring cardiac metabolism and physiology [1]. Previously described experimental systems for real-time metabolic observation in the isolated perfused heart using dDNP-NMR rely on distribution of the hyperpolarized substrate in the entire experimental medium pool (~200 ml) [2,3] in a standard Langendorff glass column, or administration to the bottom of such a system [4,5]. Another system utilized automated injectors operating in at 1 ml/min [6]. We present a modification to the perfused heart system operating at 8 ml/min and allowing fast administration of the hyperpolarized substrate [1-13C]pyruvate to the heart thereby allowing simulation of an in vivo first-pass metabolism. We show the utility of this system for determining the flux through LDH and, by using product selective excitation pulses for observation of the hyperpolarized [1-13C]lactate signal, directly determine its activity.Methods
31P and 13C NMR spectra were acquired using a 5.8 T high-resolution spectrometer (RS2D, Mundolsheim, France). Dissolution dynamic nuclear polarization (dDNP) was performed using a spin polarizer (HyperSense, Oxford instruments, Oxford, UK). Cardiac perfusion was done using a modified version of Krebs-Hensleit (KH) buffer [7]. The perfusion system was designed to allow uninterrupted flow of perfusion medium at a constant rate, controlled by a peristaltic pump. The same perfusion rate was kept also during the hyperpolarized substrate injections via a proximal bypass line.Results
LDH activity was quantified using two different acquisition approaches and analytical tools. First, we used non-selective excitation for 13C acquisition and analyzed the data with a first-order kinetic model previously described [8]. Figure 1A shows typical lactate kinetics after an injection of hyperpolarized [1-13C]pyruvic acid. The rate of the reaction was determined using a kinetic model simulation described previously [8]. This behavior was measured in a heart presenting normal cardiac energetics as determined by 31P NMR spectroscopy (Figure 1B) [9]. The rate of lactate production was dependent on the amount of ATP in the heart (Figure 1C).Discussion & Conclusions
This work demonstrates the ability to perform absolute quantification of metabolic conversion in real-time in an isolated perfused rat heart system. This setup simulates an intravenous administration of hyperpolarized substrates where the substrate is vastly diluted after the first passage through the heart. Due to the short transfer time between the spin polarizer and the myocardium the system might also allow usage of substrates with shorter T1 relaxation times. As expected, the k (pyruvate to lactate) was found to be higher when the heart contained more ATP, suggesting that this is a reliable method to quantify viable myocardial tissue. The absolute rate of myocardial LDH activity agrees well with previously published data, in which the rate of pyruvate to lactate production from pyruvate in the rat heart was found by measuring the increase in lactate concentration in the perfused medium and was found to be 238±19 µmol/g dry weight/h [10], translating to 14±1 nmol/g wet weight/s considering that the myocardium is composed of 79.4% water [11]. It is possible that our results are somewhat higher as the previous study measured net efflux of lactate to the medium while we measure all lactate produced, irrespective of subsequent metabolic fate [12]. This highlights the importance of real-time quantification of rapid enzymatic processes. In the future we hope to use this system to investigate metabolism of other substrates and investigate the LDH activity in animal models of disease.Acknowledgements
This work was supported by the European Research Council (Grant No. 338040)References
1. Bell, R. M.; Mocanu, M. M.; Yellon, D. M., Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol 2011, 50 (6), 940-950.
2. Schroeder, M. A.; Swietach, P.; Atherton, H. J.; Gallagher, F. A.; Lee, P.; Radda, G. K.; Clarke, K.; Tyler, D. J., Measuring intracellular pH in the heart using hyperpolarized carbon dioxide and bicarbonate: a 13C and 31P magnetic resonance spectroscopy study. Cardiovasc Res 2010, 86 (1), 82-91.
3. Ball, D. R.; Rowlands, B.; Dodd, M. S.; Le Page, L.; Ball, V.; Carr, C. A.; Clarke, K.; Tyler, D. J., Hyperpolarized butyrate: a metabolic probe of short chain fatty acid metabolism in the heart. Magn Reson Med 2014, 71 (5), 1663-9.
4. Merritt, M. E.; Harrison, C.; Storey, C.; Jeffrey, F. M.; Sherry, A. D.; Malloy, C. R., Hyperpolarized 13C allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proc Natl Acad Sci 2007, 104 (50), 19773-7.
5. Merritt, M. E.; Harrison, C.; Storey, C.; Sherry, A. D.; Malloy, C. R., Inhibition of carbohydrate oxidation during the first minute of reperfusion after brief ischemia: NMR detection of hyperpolarized 13CO2 and H13CO3-. Magn Reson Med 2008, 60 (5), 1029-36.
6. Mariotti, E.; Orton, M. R.; Eerbeek, O.; Ashruf, J. F.; Zuurbier, C. J.; Southworth, R.; Eykyn, T. R., Modeling non-linear kinetics of hyperpolarized [1-13C]pyruvate in the crystalloid-perfused rat heart. NMR Biomed 2016, 29 (4), 377-86.
7. Bailey, L. E.; Ong, S. D., Krebs-Henseleit solution as a physiological buffer in perfused and superfused preparations. J Pharmacol Methods 1978, 1 (2), 171-175.
8. Allouche-Arnon, H.; Hovav, Y.; Friesen-Waldner, L.; Sosna, J.; Moshe Gomori, J.; Vega, S.; Katz-Brull, R., Quantification of rate constants for successive enzymatic reactions with DNP hyperpolarized MR. NMR Biomed 2014, 27 (6), 656-62.
9. Kolwicz, S. C.; Tian, R., Assessment of cardiac function and energetics in isolated mouse hearts using 31P NMR spectroscopy. J Vis Exp 2010, (42), e2069-e2069.
10. Williamson, J. R., Effects of insulin and starvation on the metabolism of acetate and pyruvate by the perfused rat heart. Biochemical Journal 1964, 93 (1), 97-106.
11. Fernandez-Jimenez, R.; Galan-Arriola, C.; Sanchez-Gonzalez, J.; Aguero, J.; Lopez-Martin, G. J.; Gomez-Talavera, S.; Garcia-Prieto, J.; Benn, A.; Molina-Iracheta, A.; Barreiro-Perez, M.; Martin-Garcia, A.; Garcia-Lunar, I.; Pizarro, G.; Sanz, J.; Sanchez, P. L.; Fuster, V.; Ibanez, B., Effect of Ischemia Duration and Protective Interventions on the Temporal Dynamics of Tissue Composition After Myocardial Infarction. Circ Res 2017, 121 (4), 439-450.
12. Lopaschuk, G. D.; Ussher, J. R.; Folmes, C. D. L.; Jaswal, J. S.; Stanley, W. C., Myocardial fatty acid metabolism in health and disease. Physiol Rev 2010, 90 (1), 207-58.
Figures
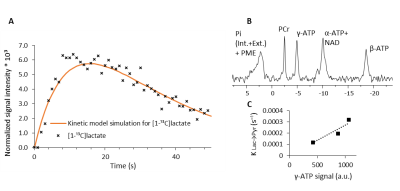
Lactate production and ATP levels.
A) The integrated intensity of [1-13C]lactate signal and its fit to the kinetic model as determined for a typical pyruvic acid injection. B) 31P spectrum of the rat heart collected immediately after the injection of hyperpolarized [1-13C]pyruvic acid. Pi (Int+Ext) + PME, intra- and extracellular inorganic phosphate and phosphomonoesters; PCr, phosphocreatine; ATP, adenosine triphosphate; NAD, nicotinamide adenine dinucleotide . C) γ-ATP signal plotted against the apparent rate constant determined using first-order kinetic model in 3 injections performed on the same rat heart. Correlation coefficient 0.9366.
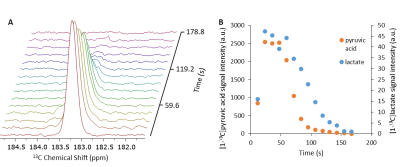
The [1-13C]lactate signal in the perfused rat heart after addition of hyperpolarized [1-13C]pyruvate observed using frequency selective pulses.
A) Time course of [1-13C]lactate signal acquired with a frequency selective pulse and nutation angles of 90 0 and 20.5 0 on [1-13C]lactate and [1-13C]pyruvate, respectively, and 11.9 s repetition time. B) Integrated signal intensity of the [1-13C]pyruvate and [1-13C]lactate signals.