3546
Parallel imaging compressed sensing for MRI-only radiation dosimetry of post-implant prostate cancer brachytherapy1Imaging Physics, MD Anderson Cancer Center, Houston, TX, United States, 2Radiation Oncology, MD Anderson Cancer Center, Houston, TX, United States, 3Diagnostic Radiology, MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Researchers recently demonstrated that both anatomical structures and implanted radioactive seeds can be visualized with high-resolution balanced steady-state free precession (bSSFP) imaging using positive-MRI-signal seed markers and an endorectal coil (ERC). However, ERC use is limited by cost, patient intolerance, and low clinical throughput. A previous preliminary study demonstrated that imaging without an ERC resulted in reduced image signal-to-noise ratio and reduced seed detection. In the current study, we investigated the feasibility of using parallel imaging compressed sensing to substantially accelerate the bSSFP acquisition and potentially enable MRI-only dosimetry of post-implant prostate cancer brachytherapy without an ERC.
Introduction
In low-dose-rate (LDR) prostate cancer brachytherapy, post-implant verification of the location of the radioactive seeds within the prostate and relative to neighboring anatomical structures is essential for ensuring favorable treatment outcomes. However, it is unclear how to optimally image the radioactive seeds in the prostate after implantation1. Researchers recently demonstrated that both anatomical structures and implanted radioactive seeds can be visualized with high-resolution balanced steady state free precession (bSSFP) imaging using positive MRI contrast seed markers2,3 and an endorectal coil (ERC). However, ERC use is limited because of its cost, patient intolerance, and negative impact on clinical throughput. In a preliminary study, we demonstrated that imaging without an ERC resulted in reduced image SNR, and, as a result, reduced seed detection and increased time to perform dosimetry4. In the current study, we investigated the feasibility of using parallel imaging compressed sensing (PICS) to accelerate bSSFP imaging. Our motivation is that the scan time savings will be used for multiple signal averages to compensate for some of the SNR loss that occurs when imaging without an ERC.Methods
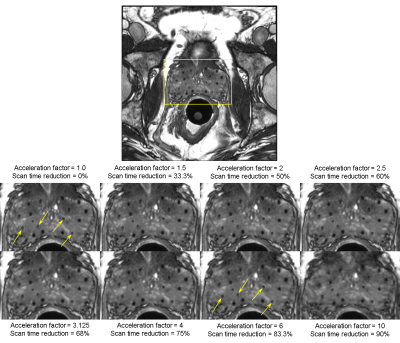
Ten patients were implanted with radioactive seeds that were stranded together with positive MR-signal seed markers. They were imaged on a Siemens 1.5T Aera scanner with a rigid two-channel ERC in combination with two 18-channel external array coils. The scan parameters were as follows: TR/TE=5.29/2.31 ms, RBW=560 Hz/px, FOV=15 cm, voxel dimensions=0.59×0.59×1.2 mm, FA=52°, and total scan time of 4-5 minutes. The patients underwent prostate brachytherapy and were scanned with a bSSFP sequence (Constructive Interference in Steady State, or CISS) on the same day. The CISS sequence provides a mix of T1 and T2 contrast with high signal-to-noise ratio (SNR), enabling visualization of radioactive seeds, seed markers, and anatomical structures in a single acquisition. The raw k-space data were collected from the scanner and retrospectively undersampled. To simulate accelerated acquisitions with increasing acceleration, we retrospectively undersampled the fully sampled datasets with seven different acceleration factors (1.5, 2, 2.5, 3.125, 4, 6, and 10) using a variable-density Poisson-disk sampling pattern. The undersampled datasets were reconstructed using a parallel imaging reconstruction algorithm (Eigenvector-based iTerative Self-consistent Parallel Imaging Reconstruction, or ESPIRiT5,6) with l1 wavelet regularization (i.e. L1-ESPIRiT) to estimate the unsampled data. The reconstructed k-space data were transferred back to the scanner and retrospectively reconstructed to generate images using the Siemens image reconstruction pipeline. A board-certified medical dosimetrist recorded the total number of directly identifiable radioactive seeds in each of the reconstructed images. The number and location of the radioactive seeds in the accelerated reconstructions were compared against those in the original, fully sampled acquisition.Results
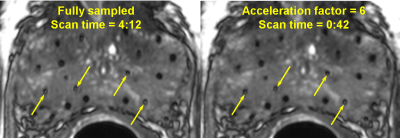
Figure 1 shows a comparison of the reconstructed images for one representative patient. In general, images reconstructed from accelerating up to a factor of 2.5 demonstrated consistently good quality with no apparent loss of SNR or depiction of anatomy compared with the fully sampled images. Image quality decreased (slight blurring, reduced seed marker contrast and overall conspicuity) with increasing acceleration factors from 3.125 to 10. Nevertheless, the dosimetrist was able to identify 96% of the radioactive seeds as well as the anatomical structures in images accelerated up to a factor of 6 (magnified view shown in Fig. 2). Compared with the original scan, the accelerated sampling corresponds to a scan time reduction of 83.3%.Discussion
Our results demonstrated that PICS can be used to substantially accelerate the bSSFP imaging of post-implant prostate cancer brachytherapy with high quality and little compromise in the detection of the radioactive seeds. If the same total scan time is maintained, we expect that acquiring six signal averages (with an acceleration factor of six) will result in an SNR boost of approximately 2.5 (~61/2), which we believe will be sufficient to compensate for most of the SNR loss from imaging without the ERC. Our study is retrospective and limited in the number of patients. Thus, a future prospective study will be needed to validate our proposed approach.Conclusion
We showed that parallel imaging compressed sensing can be used to substantially reduce the scan time of bSSFP imaging of the prostate after LDR brachytherapy. Compared with full sampling, the quality of the images from subsampled data shows minimal compromise and is sufficient for detection and localization of the vast majority of the radioactive seeds. A prospective validation of our results will have an important impact on the current standard of practice, which relies on computed tomography, by enabling MRI-only radiation dosimetry of post-implant prostate cancer brachytherapy without an ERC.Acknowledgements
No acknowledgement found.References
[1] Frank SJ, Mourtada F, Crook J, Menard C. “Use of magnetic resonance imaging in low-dose-rate and high-dose-rate prostate brachytherapy from diagnosis to treatment assessment: efining the knowledge gaps, technical challenges, and barriers to implementation,” Brachytherapy 2017;16(4):672-678.
[2] Frank SJ, Stafford RJ, Bankson JA, et al. “A novel MRI marker for prostate brachytherapy,” Int J Radiat Oncol Biol Phys 2008;71(1):5-8.
[3] Ma J, Moerland MA, Venkatesan AM, et al. “Pulse sequence considerations for simulation and postimplant dosimetry of prostate brachytherapy,” Brachytherapy 2017:16(4):743-753.
[4] Sanders J, Frank S, Bathala T, et al. “MRI-based prostate brachytherapy – imaging with and without an endorectal coil for post-implant quality assurance,” Brachytherapy 2017;16(3):S56.
[5] Uecker M, Lai P, Murphy MJ, et al. “ESPIRiT-an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA,” Magn Reson Med 2014;71(3):990-1001.
[6] Uecker M, Ong F, Tamir JI, et al. “Berkeley advanced reconstruction toolbox,” Proc Intl Soc Mag Reson Med 2015;23:2486.
Figures