3542
Accelerated volumetric renal perfusion using pseudo-continuous ASL and a 3D Fast-Spin-Echo readout with Compressed Sensing1Division of MRI Research, department of Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States, 2Global MR Applications and Workflow, GE Healthcare, Boston, MA, United States, 3Global MR Applications and Workflow, GE Healthcare, New York, NY, United States
Synopsis
While ASL is a promising technique to measure renal perfusion in multiple applications, its translation into clinical practice is still challenged by its motion-sensitivity and limited spatial coverage. In the current work, we propose an implementation of an undersampled Cartesian 3D-FSE readout with pseudo-continuous ASL labeling and Compressed-sensing reconstruction for fast whole kidney perfusion measurement. Results show that even at high acceleration factors (≈15), acceptable quality whole kidney ASL images could be obtained in less than a minute with increased motion-robustness. Furthermore, the CS acceleration enables acquiring multiple averages, providing increased coverage with similar SNR than mostly used 2D readouts.
Introduction
Arterial spin labeling (ASL) offers non-invasive measurement of kidney perfusion1 that suggests multiple potential clinical applications2,3. However, it remains a niche clinical research technique due to multiple challenges, including limited spatial coverage (2D single4/multi-slice5 readouts). A few reports of 3D-volumetric renal ASL have been presented6–8, but with long acquisition times and motion-sensitivity due to the segmented nature of 3D readouts. To accelerate acquisitions below Nyquist criteria, Compressed-sensing (CS)9 has been proposed, taking benefits of image sparsity to reconstruct pseudo-incoherently undersampled images using iterative reconstruction methods. However, its use with ASL is not well documented. In this work, we propose an implementation of an undersampled Cartesian 3D-FSE readout (providing optimal off-resonance robustness and reduced blurring compared to spiral readouts) for renal ASL. Thanks to the natural sparsity of ASL images, we hypothesize that ASL is well-suited for CS acceleration without compromising perfusion signal detection. This acceleration can be traded for speed or increased time sampling, opening interesting perspectives for 4D-CS reconstruction.Material and Methods
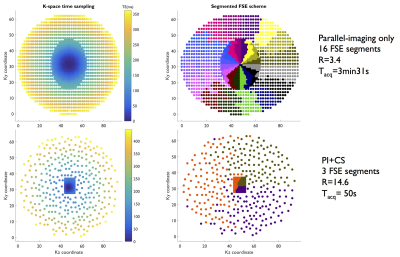
A pseudo-continuous ASL preparation was implemented with a segmented Cartesian 3D-FSE readout with an optimized elliptic-centric view-ordering ensuring short effective TE (minimizing T2-weighting and maximizing SNR). For undersampling, a ky-kz variable-density elliptic Poisson-disc sampling was implemented (fig.1).
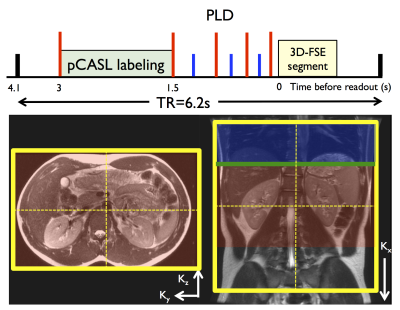
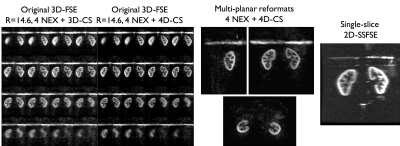
2 subjects (1M,1F,22yo) were scanned at 3T (Discovery MR750, GE Healthcare) using a 32-ch body coil. Common parameters for both parallel-imaging and PI+CS pCASL-3DFSE were: TR/TE=6200/10ms, variable refocusing flip-angle train, bandwidth=31.25kHz (echo spacing=3.2ms), FOV=340mm, 64 coronal slices (fig.2), 96x96 matrix (3.6mm isotropic resolution). Labeling was applied for 1.5s and 1.5s PLD (B1=1.4μT, Gmax/Gav=3.5/0.5mT/m)10, with interleaved label/control acquisition, background suppression and in-flow saturation. The labeling plane was positioned 8cm above the center of the imaging region to label descending aortic blood.
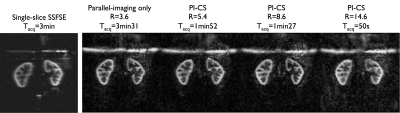
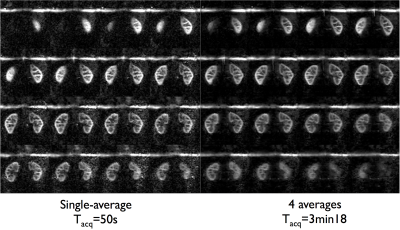
Non-CS 3D-FSE-ASL were acquired with parallel imaging (2-fold acceleration in both phase/slice directions, R=3.4), ETL=120 leading to a 16-segment FSE acquisition, Tacq=3min31 for a single-average. A M0 reference image was also acquired with PI-acceleration for coil sensitivity estimation required for CS reconstruction (Tacq=1min45) and further perfusion quantification. Total imaging time for PI-acceleration was 5min15. PI+CS 3D-FSE-ASL were acquired with an ETL=140 to minimize the number of segments acquired. Different undersampling levels were explored from R=5.7 (8 segments, Tacq=1min52) to R=14.6 (3 segments, Tacq=50s) with single-averaging. To take advantage of the acceleration provided by CS, an additional acquisition with R=14.6 and 4 repetitions (Tacq=3min18) was performed. Additionally, a coronal singe-slice single-shot-FSE-pCASL (128x128 matrix, FOV=340mm, TR/TE=6000/45ms, constant flip-angle, 14 label/control pairs, Tacq=3min) was acquired for quality comparison with the same labeling parameters. All ASL acquisitions were performed with a timed-breathing approach4.
The reconstruction was implemented using the Berkeley Advanced Reconstruction Toolbox (BART)11. First, coil-sensitivities estimation was performed on the non-undersampled M0 image using the autocalibrating ESPIRiT12 method (calibration region=323, cluster size=63, σ2cutoff=0.01 and threshold=0.8). Then, an L1-ESPIRiT reconstruction of the complex subtracted perfusion 3D k-space (dM=Mcontrol-Mlabel) was performed with sparsity in wavelet space (λ=0.001, empirically optimized). For the 4-repetitions acquisition, the same reconstruction was used with a k-space averaging prior to CS reconstruction. Additionally, a reconstruction with k-t sparsity enforcement prior to averaging was performed with spatial L1-wavelet (λ=0.001) and temporal L1-total variation (TV) (λ=0.05) regularization.
Results and discussion
CS-acceleration enables fast isotropic 3D-ASL imaging of both kidneys with comparable image quality than non-CS-accelerated up to almost 9-fold acceleration (fig.3). When comparing 2D to 3D acquisitions, the 3D ASL presents some reduced blurring due to the different refocusing trains and view-ordering used, although increased noise levels can be seen.
When focusing on the higher acceleration factors, some noise amplification appears as well as loss of smaller anatomical details as expected. Despite this, a strong perfusion signal remains and the renal cortex can be clearly identified. However, when taking benefit of the time gain to acquire multiple averages (fig.4), we can see a clear noise reduction in the resulting perfusion images with full renal coverage in the same time as 2D-SSFSE acquisitions (fig.5). Finally, the preliminary tests of 4D-CS reconstruction shows additional improvement compared to 3D-CS reconstruction especially in terms of background noise removal (enforcement of ASL signal temporal consistency).
Conclusions
We have presented here an application of CS for fast whole kidneys ASL perfusion measurement. Even at acceleration factors ≈15, good quality ASL images could be obtained under 1min, hence reducing the inherent motion-sensitivity of 3D-Cartesian-FSE ASL. This opens opportunities for improvement, such as using CS to increase SNR by averaging as shown here but also to further increase motion-robustness with 4D-CS approaches13, potentially enabling free-breathing abdominal 3D-ASL. Non-abdominal applications could also benefit from these developments, for example for brain perfusion, enabling fast multi-delay ASL using Hadamard encoding14.Acknowledgements
No acknowledgement found.References
1. Roberts, D. A. et al. Renal perfusion in humans: MR imaging with spin tagging of arterial water. Radiology 196, 281–286 (1995).
2. Mora-Gutiérrez, J. M. et al. Arterial spin labeling MRI is able to detect early hemodynamic changes in diabetic nephropathy. J. Magn. Reson. Imaging JMRI (2017). doi:10.1002/jmri.25717
3. De Bazelaire, C. et al. Arterial spin labeling blood flow magnetic resonance imaging for the characterization of metastatic renal cell carcinoma(1). Acad. Radiol. 12, 347–357 (2005).
4. Robson, P. M. et al. Strategies for reducing respiratory motion artifacts in renal perfusion imaging with arterial spin labeling. Magn. Reson. Med. 61, 1374–1387 (2009).
5. Gardener, A. G. & Francis, S. T. Multislice perfusion of the kidneys using parallel imaging: image acquisition and analysis strategies. Magn. Reson. Med. 63, 1627–1636 (2010).
6. Greer, J.S., Wang, X., Pinho, M.C., Pedrosa, I. & Madhuranthakam, A.J. Robust 3D pCASL perfusion imaging using a Cartesian Acquisition with Spiral Reordering (CASPR). in Proc. Intl. Soc. Mag. Reson. Med 3628 (2017).
7. Robson, P. M. et al. Volumetric Arterial Spin-labeled Perfusion Imaging of the Kidneys with a Three-dimensional Fast Spin Echo Acquisition. Acad. Radiol. 23, 144–154 (2016).
8. Cai, Y. et al. Diagnostic value of renal perfusion in patients with chronic kidney disease using 3D arterial spin labeling. J. Magn. Reson. Imaging 46, 589–594 (2017).
9. Lustig, M., Donoho, D. & Pauly, J. M. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 58, 1182–1195 (2007).
10. Zhao, L., Vidorreta, M., Soman, S., Detre, J. A. & Alsop, D. C. Improving the robustness of pseudo-continuous arterial spin labeling to off-resonance and pulsatile flow velocity. Magn. Reson. Med. (2016). doi:10.1002/mrm.26513
11. Uecker, M. et al. Berkeley Advanced Reconstruction Toolbox. in Proc. Intl. Soc. Mag. Reson. Med 2486 (2015).
12. Uecker, M. et al. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn. Reson. Med. 71, 990–1001 (2014).
13. Zhang, T. et al. Fast pediatric 3D free-breathing abdominal dynamic contrast enhanced MRI with high spatiotemporal resolution. J. Magn. Reson. Imaging 41, 460–473 (2015).
14. Dai, W., Shankaranarayanan, A. & Alsop, D. C. Volumetric measurement of perfusion and arterial transit delay using hadamard encoded continuous arterial spin labeling. Magn. Reson. Med. 69, 1014–1022 (2013).
Figures