3537
The influence of Sampling on Compressed Sensing accelerated high resolution Vessel Wall Imaging1University of Chinese Academy of Sciences, Shenzhen, China, 2Shenzhen Institutes of Advanced Technology, Shenzhen, China
Synopsis
This study aims to accelerate high resolution whole brain and neck vessel wall imaging using combined Compressed Sensing and Parallel Imaging. The influence of sampling choices with different control of sampling density and randomness on CSPI reconstruction were investigated on retrospectively and prospectively accelerated in vivo datasets, with emphasis on the sharpness of vessel wall borders which was critically demanded by detecting potential vessel wall thickening and atherosclerotic plaques for ischemic stroke patients.
Introduction
Compressed Sensing combined with parallel imaging (CSPI) 1 is highly demanded to accelerate the high resolution 3D MR vessel wall imaging 2 for ischemic stroke patients since a state-of-the-art scan usually requires 10 minutes. However, the frequently occurred blurring artifacts of CS acceleration may hinder its application in vessel wall imaging since sharply depicting wall borders is clinically demanded. Thus, this work aims to investigate the influence of sampling schemes with different control of density and randomness 3,4 on CSPI accelerated vessel wall imaging with emphasis on the wall border sharpness.Method
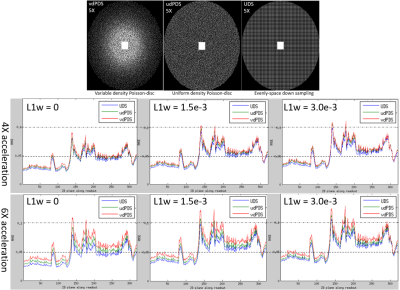
Sampling and reconstruction: Three CSPI sampling choices with different sampling density and randomness were compared: (a) uniform density Poisson-disc sampling (udPDS) generated randomly distributed sampling points that are tightly-spaced, but no closer to each other in two dimensions 3 , (b) variable density Poisson-disc sampling (vdPDS) was achieved by enforcing a probability density function (PDF) into udPDS which was defined as: $$$P(k_x,k_y) = max(1-\sqrt{k_x^2 + k_y^2}, 0)^d$$$, where $$$k_x$$$ and $$$k_y$$$ denotes the k-space coordinates scaled between -1 and 1, and the power $$$d$$$ determines the sampling density variation with lower values result in denser sampling in the k-space center, (c) Uniformly spaced downsampling (UDS) was traditionally used for parallel imaging, and might achieve better result with CSPI reconstruction 5. Elliptical scanning and a fully sampled k-space center of size 24 x 24 were enforced for additional acceleration and PI calibration separately. Figure 1(a) demonstrated udPDS, vdPDS with power $$$d=3$$$, and UDS to achieve 5X acceleration. All accelerated datasets were reconstructed by L1-SPIRiT.
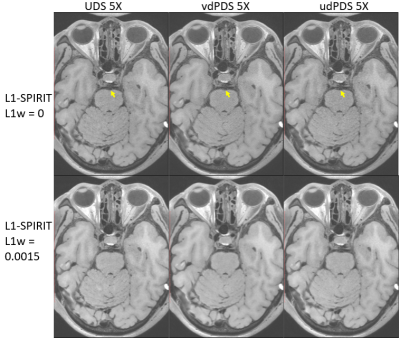
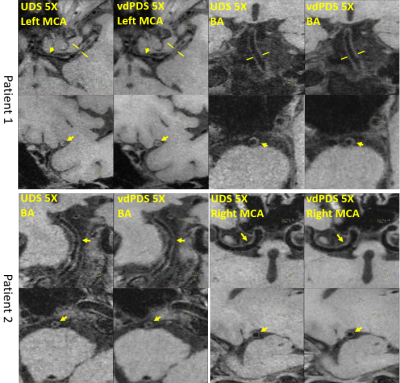
In-vivo study: In-vivo experiments were IRB approved with written informed consents obtained from all subjects. All measurements were performed on a 3T scanner (Siemens Tim Trio, Germany) with a 32-channel head and neck coil. (a) Retrospective acceleration: a fully sampled dataset was firstly acquired from a healthy volunteer (female, 26 years old) with the following imaging parameters: 3D sagittal orientation, field of view (FOV) = 200 x 200 x 128 mm, isotropic resolution = 0.62 $$$mm^3$$$, matrix size = 320 x 320 x 208, TE/TR/TI = 12/800/100 ms, echo spacing = 5.12 ms, echo train length (ETL) = 41, and scan time = 24 min. Then the full data was retrospectively downsampled by udPDS, vdPDS, and UDS separately at 4X and 6X accelerations. The reconstruction was conducted using C++ implemented 2D L1-SPIRiT along readout dimension running on a workstation equipped with an Intel Xeon E5-2698 CPU and 256 GB RAM. Finally, the reconstructed images were compared with reference by Root-Mean-Square-Error (RMSE). (b) Prospective acceleration: firstly, three downsampling schemes were prospectively implemented into the 3D SPACE sequence for vessel wall imaging. Then three patients were recruited, and three 5X accelerated datasets using different downsampling schemes were acquired from each patient. Additional imaging parameters were: 3D sagittal orientation, FOV = 212 x 180 x 140 mm, matrix size = 384 x 326 x 256, isotropic resolution = 0.55 $$$mm^3$$$, TE/TR/TI = 8.8/850/100 ms, echo spacing = 4.4 ms, and ETL = 48. The 5X prospective acceleration reduced the scan time of whole brain and neck vessel wall imaging into 5 minutes. The accelerated scans was reconstructed online by the same L1-SPIRiT as used in the retrospective experiments. Finally, the intracranial vessel walls were reconstructed by multiplanar reconstruction (MPR), and were compared between different downsampling schemes by visual inspection of wall border sharpness.
Results
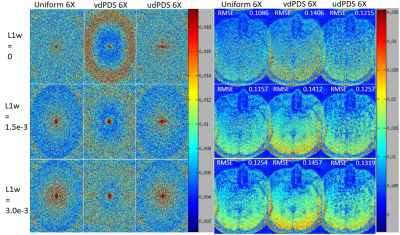
Figure 1 shows the RMSE plots along the readout dimension of L1-SPIRiT reconstructions of 4X and 6X retrospectively accelerated datasets, and demonstrates that vdPDS lead to larger reconstruction errors than udPDS and UDS. Figure 2 illustrates the reconstruction errors in final k-space and image of a represented 2D slice along readout. Results of vdPDS suffered from more significant noise amplification due to higher reduced sampling in the k-space periphery. The utilization of CS with a moderate L1 weight could alleviate the PI amplified noise without sacrificing image sharpness as demonstrated in Figure 2 and 3. However, CS was unable to recovery image details lost due to insufficient sampling in high frequency of k-space. Thus, the prospective vdPDS acceleration suffers from more obvious wall border blurring than UDS as demonstrated in Figure 4 given the same reconstruction and acquisition time.Discussion
This work investigated the influence of sampling schemes with different control of sampling density and randomness on CSPI accelerated high resolution vessel wall imaging. Results of variable density Poisson-disc sampling suffered from larger global reconstruction error and more significant local vessel wall border blurring than uniform density sampling. A moderate L1 regularization could alleviate noise without scarifying image sharpness, but was unable to recovery image details deteriorated by insufficient sampling in k-space periphery with vdPDS.
Acknowledgements
This work is supported in part by the National Natural Science Foundation of China (61471350) and the Science and Technology Program of Guangdong (2015A020214019).References
- Lustig M, Pauly JM. SPIRiT: Iterative Self-consistent Parallel Imaging Reconstruction From Arbitrary k-Space. Magn Reson Med. 2010, 64:457-471.
- Lei Zhang, Na Zhang, Jun Wu, Xin Liu, Yiu-Cho Chung. High resolution simultaneous imaging of intracranial and extracranial arterial wall with improved cerebrospinal fluid suppression. Magnetic Resonance Imaging. 2017, 44:65-71.
- Vasanawala SS, Alley MT, Hargreaves BA, Barth RA, Pauly JM, Lustig M. Improved pediatric MR imaging with compressed sensing. Radiology. 2010, 256:607-616.
- Duanduan Liu, Dong Liang, Xin Liu, Yuanting Zhang. Under-sampling trajectory design for Compressed Sensing MRI. 34th Annual International Conference of the IEEE EMBS, 2012, pp 73-76.
- Weller DS, Polimeni JR, Grady L, Wald LL, Adalsteinsson E, Goyal VK. Denoising sparse images from GRAPPA using the Nullspace method. Magn Reson Med. 2012, 68:1176-1189.
Figures