3536
Feasibility of High Spatial and Temporal Resolution Breast DCE-MRI using Radial Acquisition with Data-Driven Model Consistency Condition Reconstruction1Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Carbone Cancer Center, University of Wisconsin-Madison, Madison, WI, United States, 4Global MR Applications & Workflow, GE Healthcare, Madison, WI, United States
Synopsis
Dynamic contrast-enhanced (DCE) MRI using conventional Cartesian sampling is used in routine clinical practice due to its high sensitivity for breast cancer. However, ghosting artifacts caused by cardiac motion can obscure the axilla, making interpretation of this area more difficult and potentially obscuring findings. Radial acquisitions are less motion sensitive due to more frequent sampling of the center of k-space and prior work has suggested these methods for breast MRI. In this study, we report results from a reader study to assess image quality of a 3D stack-of-stars radial acquisition compared with Cartesian imaging for breast MRI.
Introduction
Dynamic contrast enhanced (DCE)-MRI with conventional Cartesian acquisition has become a powerful tool for breast cancer assessment (1). Further, DCE-MRI offers the promise of deriving pharmacokinetic parameters that could distinguish benign and malignant lesions (2). However, this requires high temporal resolution data to accurately represent rapid physiological processes (3) necessitating reconstruction from incomplete data. Standard approaches such as view-sharing can result in temporal blurring leading to inaccuracies in pharmacokinetic analysis (4). Additionally, cardiac motion results in ghosting artifacts for Cartesian acquisition that may obscure the axilla and reduce the ability to accurately characterize temporal enhancement. Another approach to reconstructing incomplete data is provided by compressed sensing (CS) (5) which has recently been extended to breast DCE-MRI (6). CS provides accurate reconstruction of images if they adopt a sparse representation in some basis and if k-space data is undersampled with a randomized pattern producing incoherent artifacts. Sparse representation of dynamic image data can be obtained in the temporal dimension and radial acquisition satisfies the second condition while simultaneously providing robustness to motion. In this study, we determine the feasibility of combining radial acquisition with data-driven model consistency condition (7) for high spatial and temporal resolution breast DCE-MRI.Methods
Simulation: A time-resolved acquisition was simulated by using a digital breast phantom with the matrix size 448x448x10 that included cardiac motion (8). Simulated lesions were placed in the fibroglandular tissue of the right breast and in the axillary tail region bilaterally. Different pharmacokinetic parameters (ktrans, Ve and Vp) were assigned to each lesion to model contrast kinetics. Cardiac motion was simulated using 1.3 sec/beat with 11 cardiac phases and the signal intensity in the heart was modulated using a simulated arterial input function (9). The digital phantom was used to simulate Cartesian and golden-angle radial acquisitions. Cartesian data were reconstructed for 16 image frames at 44 s temporal resolution. The uniform coverage of golden angle sampling scheme allowed for reconstruction of radial k-space data at two different temporal resolutions, 11 and 5.5s, corresponding to 16 and 8 projections per time frame respectively. Both of these time series were reconstructed using model consistency condition (MOCCO) with the underlying model learned from a low-resolution image series estimate from the fully-sampled center of k-space.
In-vivo: A normal volunteer was imaged during gadolinium contrast injection (gadobenate dimeglumine, Multihance; Bracco Inc, Milan, Italy) on a clinical 3T MRI system (Signa PET/MR, GE Healthcare, Waukesha, WI) using an 8-channel breast coil (GE Healthcare) for this IRB approved, HIPAA compliant study. A 3D stack-of-stars golden-angle gradient echo imaging sequence (TE/TR= 5.876/2.796 ms , FOV= 380 x 380) was used to collect 256 radial projections at each z-phase encode with matrix size 448×448×142. The time series was reconstructed with 16 projections/frame using MOCCO, resulting in 11 s temporal resolution.
Results
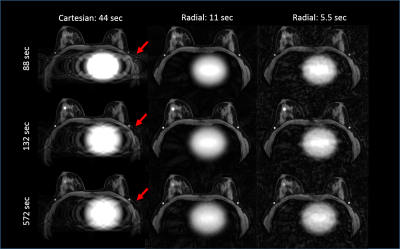
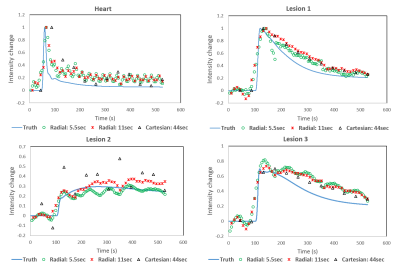
Simulations: The simulated Cartesian acquisition demonstrates well-visualized structures in the breasts, however phase-ghosting due to the cardiac motion is evident in the left axilla (Figure 1) which alters the wash-out intensity in a simulated lesion in the axillary tail (Figure 2, lesion2). Using the radial acquisition at 11s temporal resolution (acceleration factor R=44) shows similar image quality in the breasts compared with the fully sampled Cartesian acquisition. Additionally, the axilla was well depicted. At higher accelerations (R=88) streaking artifacts remain despite advanced reconstruction, however, they do not produce a detrimental effect on time-signal curves, which are shown for each of the simulated lesions and reconstructions in Figure 2. Time curves from the Cartesian acquisition do not show substantial deviations from the input curves for lesions 1 and 3. However, strong signal fluctuations are observed in lesion 2 due to the cardiac ghosting.
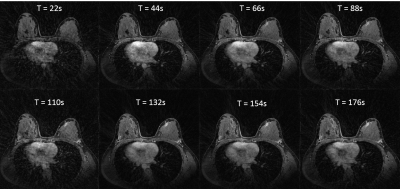
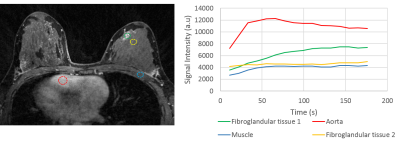
In-vivo: Results from the volunteer study provide visualization of the contrast kinetics without evidence of cardiac ghosting (Figure 4). Individual ROIs placed on specific tissues of interest show the ability to capture different temporal dynamics. Note there is increased streaking at the time-frame corresponding to contrast arrival and high signal present in the heart (Figure 4).
Discussion and conclusions
In this study, we demonstrate the potential of using radial acquisition with MOCCO reconstruction to generate high temporal and spatial resolution breast images. Both simulation and in-vivo data allowed for an effective temporal resolution of 11 s, equivalent to 44x angular undersampling. The simulation results also demonstrated the radial approach could overcome the impact of cardiac motion and ghosting on the assessment of contrast kinetics compared to the Cartesian acquisition. Although at higher acceleration factors with frame rates of 5 s/frame the reconstructed images suffered from undersampling artifacts, the temporal waveforms of interest were not affected.Acknowledgements
The authors wish to acknowledge GE Healthcare and the RSNA Research and Education Foundation for their research support.References
1. Mei-Lin W. Ah-See et al, Early Changes in Functional Dynamic Magnetic Resonance Imaging Predict for Pathologic Response to Neoadjuvant Chemotherapy in Primary Breast Cancer, Clinical Cancer Research, Vol.14, 2008
2. Partridge, S. C., Stone, K. M., Strigel, R. M., DeMartini, W. B., Peacock, S., & Lehman, C. D. (2014). Breast DCE-MRI: influence of postcontrast timing on automated lesion kinetics assessments and discrimination of benign and malignant lesions. Academic radiology, 21(9), 1195-1203.
3. Henderson et al. Temporal sampling requirements for the tracer kinetics modeling of breast disease, Magn. Reson. Imaging, 16(9), 1998:1057-73
4. Tsao, J., & Kozerke, S. (2012). MRI temporal acceleration techniques. Journal of Magnetic Resonance Imaging, 36(3), 543-560.
5. E. J. Candès. Compressive sampling. Proceedings of the International Congress of Mathematicians, Madrid, Spain, 2006.
6. Heacock, L., Gao, Y., Heller, S. L., Melsaether, A. N., Babb, J. S., Block, T. K., ... & Moy, L. (2017). Comparison of conventional DCE‐MRI and a novel golden‐angle radial multicoil compressed sensing method for the evaluation of breast lesion conspicuity. Journal of Magnetic Resonance Imaging, 45(6), 1746-1752.
7. Velikina, J. V., & Samsonov, A. A. (2015). Reconstruction of dynamic image series from undersampled MRI data using data‐driven model consistency condition (MOCCO). Magnetic resonance in medicine, 74(5), 1279-1290.
8. Henze LC, Moran CJ, Smith MR, Kelcz F, Samsonov A, Fain SB, Block WF. Digital Breast Phantom for Evaluating Dynamic Accelerated Imaging Methods. Proceedings of the 18th ISMRM Scientific Meeting 2010. Stockholm Sweden, 2010.
9. Barboriak DP, MacFall JR, Viglianti BL, Dewhirst Dvm MW. Comparison of three physiologically-based pharmacokinetic models for the prediction of contrast agent distribution measured by dynamic MR imaging. J Magn Reson Imaging 2008;27(6):1388-98.
Figures