3534
Compressive Sensing Reconstruction for Multi-Contrast Data with Unequal Acceleration Rates1Department of Advanced Sensing Research Programs, ASELSAN Research Center, Ankara, Turkey, 2School of Psychology / CUBRIC, Cardiff University, Cardiff, United Kingdom, 3Electrical and Electronics Engineering, Bilkent University, Ankara, Turkey, 4National Magnetic Resonance Research Center (UMRAM), Bilkent University, Ankara, Turkey, 5Neuroscience Program, Bilkent University, Ankara, Turkey
Synopsis
In multi-contrast acquisitions, a critical concern is whether to distribute undersampling uniformly or unequally across contrasts, as scan times and SNR typically vary among sequences. This study investigates a compressive sensing framework in jointly reconstructing multi-contrast data with unequal acceleration rates. Using in-vivo and numerical datasets, the total scan time was fixed and acceleration factors were varied between protocols. The results suggest using lower acceleration rates for protocols with higher-SNR and shorter duration, and higher rates for protocols with lower-SNR and longer duration improves image quality, even in the highly accelerated contrast. The method was also compared to seven state-of-the-art methods from the literature.
Introduction
This study investigates a compressive sensing (CS) framework in jointly reconstructing multi-contrast MRI data with unequal acceleration rates.
Multi-contrast images are commonly acquired to maximize complementary tissue information, albeit at the cost of longer scans. CS can be used to accelerate scans1, and image quality improvements have recently been demonstrated through joint reconstruction of multi-contrast images2-4. Because scan times and SNR typically vary among sequences in a multi-contrast protocol, a critical concern is whether to distribute undersampling uniformly or unequally across contrasts, which remains a topic understudied.
Here, we investigate the effects of unequal acceleration among contrasts on reconstruction quality on in-vivo and numerical datasets consisting of three-contrasts (T1-, T2-, PD-weighted) by fixing the total scan time and varying acceleration factors between protocols. Then, we compare the efficacy of common reconstruction methods for multi-contrast CS-MRI, along with a joint reconstruction framework that we recently proposed5,6. We investigate the reliability of these methods against leakage of uncommon features across contrasts, a major concern for joint reconstruction7.
Methods
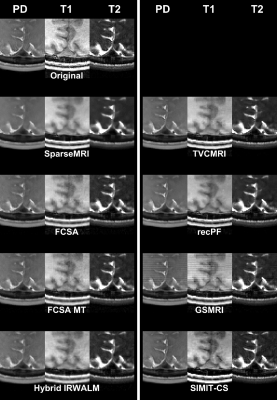
We recently proposed a joint reconstruction framework (SIMIT-CS5,6: Simultaneous use of Individual and Mutual Information Terms in Compressive Sensing) for CS that simultaneously uses individual and joint regularization terms across multiple contrasts. While joint terms (Group-L1-Sparsity2 and Color-TV8) improve image quality by better utilizing shared information among contrasts, individual terms (L1-sparsity and TV) increase sensitivity to unique information in each contrast to prevent leakage of features across contrasts. Here we used SIMIT-CS to jointly reconstruct multi-contrast datasets and compared SIMIT-CS with an implementation that only uses individual terms (Hybrid-IRWALM9), and six other state-of-the-art reconstructions methods (SparseMRI1, TVCMRI10, recPF11, GSMRI2, FCSA12, FCSA-MT4).
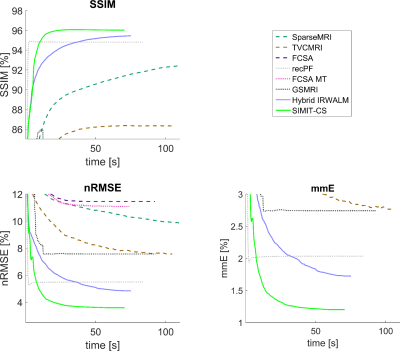
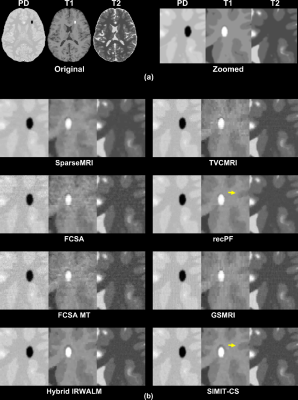
Simulated Data: Simulations were performed using Matlab (Mathworks Inc.,Natick,MA) with a numerical-dataset generated from a segmented brain phantom13. Regularization parameters were optimized for each method (interval-search algorithm seeking maximum structural-similarity-index [SSIM] for 3-fold accelerated 5-contrast numerical-dataset [PD-T1-T2-FLAIR-STIR]), and used for all reconstructions hereafter. First, SIMIT-CS and Hybrid-IRWALM were compared in a 3-contrast/two-protocol (PD/T2 acquired together as early/late echoes with TR=2750ms and T1 with TR=550ms) acquisition by fixing the total scan time TA=6:30minutes and varying acceleration factors among protocols. Then, artificial features were added to a subset of images to test methods against leakage-of-features. Reconstructions are given for R_T1=6, R_PD=R_T2=1.9 (TA=6:30minutes). Reconstruction quality was assessed via SSIM, mmE (mean-magnitude error), and nRMSE (normalized root-mean-squared error).
In-vivo Data: Experiments were performed on a 3T scanner (Siemens Healthineers,Erlangen,Germany) using a 32-channel receiver (approval of local ethics committee and informed consent of the volunteer acquired). Data were undersampled retrospectively, reconstructed separately for each channel and then combined14. Two protocols were used: MP-RAGE (TR=2000ms) for T1-data, and spin-echo (TR=750ms) for PD-/T2-data (acquired together as early/late echoes), with a total scan time of 8:38minutes (PHASExREAD resolution=192x256). SIMIT-CS and Hybrid-IRWALM were compared for various retrospective acceleration rates yielding TA=3:30minutes. In-vivo reconstructions are given for R_PD=R_T2=2, R_T1=4 for all methods.
Undersampling Masks: Two-dimensional masks were generated in two phase-encode dimensions. One-eighth of the k-space was fully-sampled. Variable-density random sampling was performed via an nth-order decay with k-space radius, where n=max(R-2,3) and R is the acceleration rate. Masks were different across contrasts, but the same among methods.
Results and Discussion
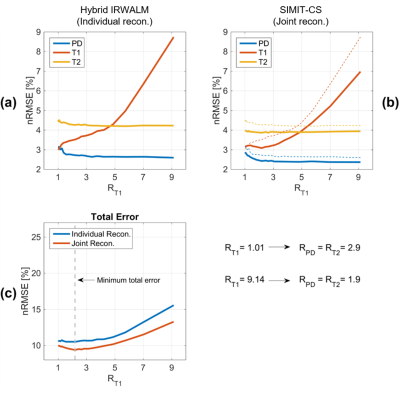
Figure 1 shows that for a wide range of acceleration factors with TA=6:30minutes, SIMIT-CS yields lower nRMSE than Hybrid-IRWALM. With all contrasts having comparable SNR, the minimum total error was acquired at a uniform acceleration rate of 2.2 across contrasts.
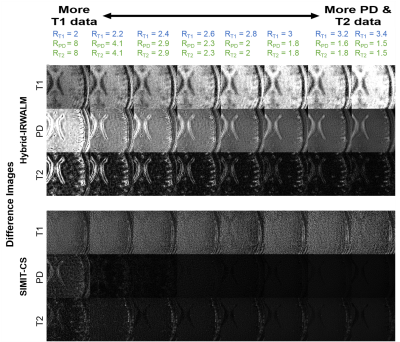
A similar analysis was made for the in-vivo data (TA=3:30minutes, R_PD=R_T2 varied between 1.5 and 8) with much different results. Remarkably, the difference images for T1 (Fig. 2) were almost unaffected by R_T1 for SIMIT-CS. There are two reasons for this behavior, i) due to the difference in TR’s, a total of 16 datapoints were acquired for PD- and T2-data for each 3 datapoints skipped for T1-data; ii) PD- and T2-data had much higher SNR (5-fold and 3.6-fold, respectively) compared to T1-data in our experiments. SIMIT-CS can utilize this surge of additional information with higher-SNR to make up for skipped T1-datapoints. These results suggest that higher acceleration rates for slower/lower-SNR sequences and lower rates for faster/higher-SNR sequences should be used to improve image quality across all contrasts. Here, the total error across all contrasts was minimum for R_T1=2.8 and R_PD=R_T2=2. A similar trend towards unequal acceleration rates was observed in the numerical-dataset when the SNR level of the T1-weighted image was relatively low compared to PD and T2 (not shown).
SIMIT-CS reconstructed higher quality images than reference methods for the numerical-dataset (Fig. 3), and without any leakage-of-features across contrasts (Fig. 4). In-vivo scans also show benefits of joint reconstruction. SIMIT-CS yielded visually sharper images, with another joint method FCSA-MT having the closest performance (Fig. 5).
Acknowledgements
The authors would like to thank the authors of SparseMRI1, TVCMRI10, recPF11, GSMRI2, FCSA12 and FCSA-MT4 for sharing their algorithms online.References
1. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med 2007;58(6):1182-1195.
2. Majumdar A, Ward RK. Joint reconstruction of multiecho MR images using correlated sparsity. Magnetic Resonance Imaging 2011;29(7):899-906.
3. Bilgic B, Goyal VK, Adalsteinsson E. Multi-contrast reconstruction with Bayesian compressed sensing. Magn Reson Med 2011;66(6):1601-1615.
4. Huang J, Chen C, Axel L. Fast multi-contrast MRI reconstruction. Magnetic Resonance Imaging 2014;32(10):1344-1352.
5. Kopanoglu E, Gungor A, Kilic T, Saritas EU, Cukur T, Guven HE. Joint Reconstruction of Multi-Contrast Images: Compressive Sensing Reconstruction using both Joint and Individual Regularization Functions. 2017; Honolulu, HI, USA. p 3875.
6. Kopanoglu E, Gungor A, Kilic T, Saritas EU, Cukur T, Guven HE. SIMIT-CS – Simultaneous use of Individual and Mutual Information Terms in Compressive Sensing: Joint Reconstruction of Multi-Contrast MRI Acquisitions. (submitted).
7. Knoll F, Holler M, Koesters T, Otazo R, Bredies K, Sodickson D. Joint MR-PET reconstruction using a multi-channel image regularizer. IEEE Transactions on Medical Imaging 2016;PP(99):1-1.
8. Blomgren P, Chan TF. Color TV: total variation methods for restoration of vector-valued images. Image Processing, IEEE Transactions on 1998;7(3):304-309.
9. Guven HE, Gungor A, Cetin M. An Augmented Lagrangian Method for Complex-Valued Compressed SAR Imaging. IEEE Transactions on Computational Imaging 2016;2(3):235-250.
10. Shiqian M, Wotao Y, Yin Z, Chakraborty A. An efficient algorithm for compressed MR imaging using total variation and wavelets. 2008 23-28 June 2008. p 1-8.
11. Yang J, Zhang Y, Yin W. A Fast Alternating Direction Method for TVL1-L2 Signal Reconstruction From Partial Fourier Data. IEEE Journal of Selected Topics in Signal Processing 2010;4(2):288-297.
12. Huang J, Zhang S, Metaxas D. Efficient MR image reconstruction for compressed MR imaging. Medical Image Analysis 2011;15(5):670-679.
13. Aubert-Broche B, Griffin M, Pike GB, Evans AC, Collins DL. Twenty new digital brain phantoms for creation of validation image data bases. IEEE Trans Med Imaging 2006;25(11):1410-1416.
14. Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med 2000;43(5):682-690.
Figures