3524
Uniform Combined Reconstruction (UNICORN) of Multi-channel Surface-coil Data at 7T without use of a Reference Scan1Siemens Healthineers, Rochester, MN, United States, 2Siemens Healthineers, Austin, TX, United States, 3Siemens Healthineers, Portland, OR, United States, 4Siemens Healthineers, Erlangen, Germany, 5Mayo Clinic, Rochester, MN, United States
Synopsis
An algorithm for correcting the intensity non-uniformity in MR images without the use of a calibration/reference scan was proposed and its efficacy was demonstrated at ultra-high-field in musculoskeletal MRI. The algorithm was shown to provide better sensitivity in the inferior/superior regions of the knee compared to state-of-the-art inhomogeneity correction filters. Without the use of a reference scan, the algorithm was also shown to provide image uniformity equivalent to calibration based methods.
Purpose
Uniformity in MR images impacts diagnosis, quantification and automation. Intensity inhomogeneity in MRI is due to multiple factors including the main magnetic-field (B0), transmit-field (B1) and receive (surface) coil-sensitivity. Various approaches for correcting the effect of receive coil-sensitivities on MRI uniformity were proposed (1–8). Among these, the methods that employ a reference or calibration scan are used clinically more often due to factors such as consistent through-slice intensity profile and preservation of quantitative accuracy in dynamic scans (9). The reference scan (often from the body coil) used for uniformity correction may be non-uniform or not available at ultra-high-field such as 7T.
Bias-field correction methods (1,3,10) that are often used for uniformity correction at field strengths such as 3T and 1.5T were shown to be insufficient at ultra-high-field (8,11). Additionally, most of the methods used for uniformity correction were developed for or evaluated extensively in the brain (5,6,8).
The purpose of this work is to develop an anatomy independent algorithm that does not need a reference scan for correcting the non-uniformity caused by the receive-coils in MR images and evaluate its performance in musculoskeletal MRI at ultra-high-field.
Methods
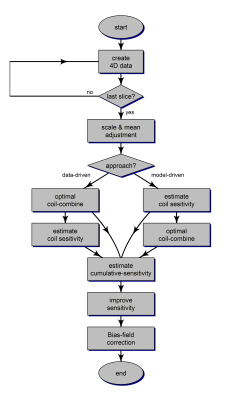
Flow-chart of the proposed algorithm for Uniform Combined Reconstruction (UNICORN) of multi-channel receive-coil data is shown in Figure 1. Receive-coil induced non-uniformity was assumed to be multiplicative and of low spatial frequency. The proposed algorithm has the following steps
- Create 4D data using all receive-coil and slice information for the imaging volume (3 spatial dimensions (x, y and z) and 1 coil dimension)
- Optimize the scale and mean of the individual coil data
- Perform optimized combination of the coil data
- Estimate individual coil-sensitivity
- Compute cumulative coil-sensitivity over the volume
- Enhance the sensitivity of the algorithm using cumulative coil-sensitivity
- Compensate any residual intensity inhomogeneity from factors such as B1 using bias-field correction methods.
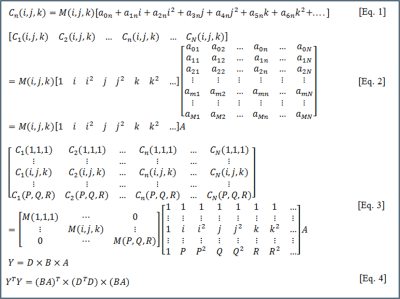
Steps 3 and 4 are performed in sequence for a data-driven approach. When a model-driven approach is used, coil-sensitivities are estimated first assuming polynomial model for coil-sensitivity and coil-combination is performed subsequently. Figure 2 shows the procedure for estimating the coil-sensitivities using $$$C_n(i,j,k)$$$, the signal measured from location $$$(i,j,k)$$$ by the n-th among the N receive-coils and $$$M(i,j,k)$$$ is the "true" intensity of the voxel.
Imaging was performed using a single-channel transmit, 28 channel receive knee coil on a 7T MRI system (Magnetom Terra, Siemens Healthineers, Erlangen, Germany) using a turbo-spin-echo pulse sequence on 5 subjects. Additionally, 5 subjects were scanned on a 3T MRI system (Magnetom Skyra, Siemens Healthineers, Erlangen, Germany) using both single-channel transmit, 15 channel receive and single-channel transmit, single-channel receive knee coils. Single-channel receive data is expected to have more uniform receive-sensitivity throughout the imaging volume as compared to multi-channel receive and was used as a benchmark for uniformity comparison at 3T.
Uniformity correction was performed using N3 and UNICORN algorithms at 7T and using a reference based method and UNICORN at 3T.
Image uniformity was evaluated and compared between different methods by two experienced radiologists.
Results
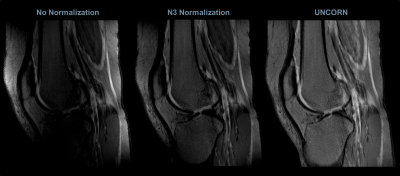
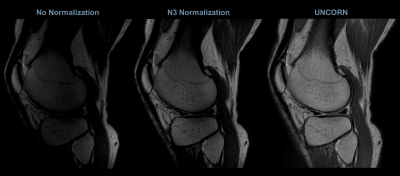
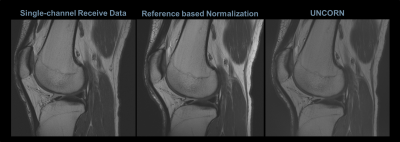
Figures 3, 4 and 5 compare the uniformity of sagittal knee images reconstructed using the UNICORN algorithm with the results from N3 normalization (at 7T) and calibration based normalization (at 3T) respectively.
Improved uniformity in the anterior/posterior regions and better sensitivity at the inferior/superior regions of the knee can be observed with the proposed algorithm at 7T.
Similar uniformity between single-channel receive-coil data and UNICORN was observed for the 3T knee images.
The results demonstrate that the intensity non-uniformity caused by receive-coils (observed as hyper-intensities proximal to the receive-coils and as hypo-intensities near the center of the field-of-view) was corrected to a significant extent by the UNICORN algorithm without the use of a reference scan.
Discussion and Conclusions
Uniform reconstruction of multi-channel surface-coil data without use of a reference scan is essential for clinically acceptable image quality on systems such as 7T MRI that do not or cannot acquire a reference scan. The UNICORN method does not need a reference scan because the coil-sensitivities are estimated from the multi-channel data and decoupled to obtain a uniform image/volume.
The proposed algorithm provides sensitivity uniformly throughout the imaging volume because of the use individual channel data for uniformity correction and enhancing the sensitivity at all regions of the imaging volume using the cumulative coil-sensitivity map. However, bias-field correction methods, such as N3, typically correct the non-uniformity in a coil-combined image thus not availing the coil specific non-uniformity information for normalization.
In summary, the UNICORN algorithm has potential to achieve uniformity without the use of a reference scan at ultra-high-field and lower field strengths.
Acknowledgements
We thank our colleagues Heiko Meyer, Wilfried Landschuetz and Andreas Schaefer for their helpful discussions and feedback.References
1. Boyes RG, Gunter JL, Frost C, Janke AL, Yeatman T, Hill DLG, et al. Intensity non-uniformity correction using N3 on 3-T scanners with multichannel phased array coils. Neuroimage. 2008;39(4):1752–62.
2. Fuderer M, Duijndam AJ, Jones AA, Harder JM Den, Rozijn TH. Intensity correction at high field MRI by filtering of the reference scan. In: Intl Soc Mag Reson Med 11. 2004. p. 2145.
3. Tustison NJ, Cook PA, Gee JC. N4ITK: Improved N3 Bias Correction. IEEE Trans Med Imaging. 2010;29(6):1310–20.
4. Sled JG, Zijdenbos a P, Evans a C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97.
5. Belaroussi B, Milles J, Carme S, Zhu YM, Benoit-Cattin H. Intensity non-uniformity correction in MRI: Existing methods and their validation. Med Image Anal. 2006;10(2):234–46.
6. Pernu F, Vovk U, Pernus F, Likar B. A Review of Methods for Correction of Intensity Inhomogeneity in MRI. IEEE Trans Med Imaging [Internet]. 2007;26(3):405–21.
7. Mikheev A, Rusinek H, Wiggins G. Non-uniformity normalization using 3D Canny edges and Legendre polynomial approximation of the bias field: validation on 7T T1W brain images. In: Proc Intl Soc Mag Reson Med 21. 2013. p. 2695.
8. Luesebrink F, Mettern H, Sciarra A, Speck O. Quantitative and Qualitative Evaluation of Bias Field Correction Methods. Proc Intl Soc Mag Reson Med. 2017;1451.
9. Ogasawara G, Inoue Y, Matsunaga K, Fujii K, Hata H, Takato Y. Image Non-Uniformity Correction for 3-T Gd-EOB-DTPA-Enhanced MR Imaging of the Liver. Magn Reson Med Sci. 2017;16:115–22.
10. Li C, Gore JC, Davatzikos C. Multiplicative intrinsic component optimization (MICO) for MRI bias field estimation and tissue segmentation. Magn Reson Imaging 2014;32(7):913–23.
11. Mikheev A, Rusinek H, Wiggins G. Non-Uniformity Correction at 7T Using High-Order Legendre Polynomial Approximation of the Bias Field. In: ISMRM SCIENTIFIC WORKSHOP on Ultra High Field MRI:, At Noordwijk aan Zee, The Netherlands. 2013.
Figures