3509
Improved Parallel Imaging Reconstruction of EPI using Inversely Distortion Corrected FLASH as Calibration Data1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China
Synopsis
For parallel imaging reconstruction of EPI, EPI based calibration scan may suffer from ghost artifacts, whereas non-EPI based calibration scan such as FLASH cannot provide consistent geometric distortion. In this study, we propose to employ dual-echo FLASH as the calibration scan, such that B0 field maps can be derived to match FLASH images to EPI images and the reconstruction artifact related to inconsistent distortion can be minimized.
Introduction
For parallel imaging reconstruction of echo-planar imaging (EPI), it is generally allowed to acquire the calibration data using either EPI or non-EPI sequences such as FLASH. Compared with EPI based calibration, FLASH based calibration can offer higher SNR images and is free from ghost artifacts. However, it cannot provide consistent geometry distortion for ideal pixel matching, and thus may cause artifacts in areas with large field inhomogeneity1-3. In this study, we propose to employ dual-echo FLASH as the calibration scan, such that B0 field maps can be derived to match the FLASH images to EPI images and the reconstruction artifacts related to inconsistent distortion can be minimized.Methods
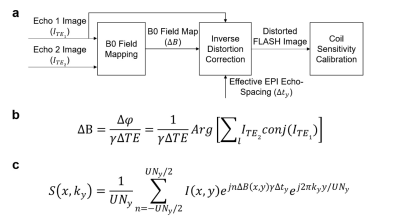
Dual-echo FLASH data are used for both field mapping and coil sensitivity calibration as illustrated in Fig 1a. First, the images of the two echoes are upsampled (×2) by zero-padding in k-space. Then image phase difference between the two echoes are calculated, unwrapped, and filtered by a 2-D median filter (kernel size 7×7)4,5. From the filtered phase difference maps, the B0 field map is calculated (Fig 1b), and subsequently masked based on the magnitude image and dilated to remove interference from signal void regions.
Based on the equation in Fig 1c and the effective echo-spacing of EPI data, the geometric distortion is enforced to the first echo of FLASH data in x-ky space4,5. After such inverse distortion correction, the FLASH images are downsampled to their original matrix size and used to calculate GRAPPA weights or coil sensitivity maps.
Experiments: Human brain data were acquired on a 3T Philips scanner with 32-channel coils. A healthy volunteer was scanned with standard shimming at in-plane acceleration factor (R) = 2. For dual-echo FLASH, TE = 6/12 ms, TR = 410 ms, flip angle =30 degrees, slice thickness = 4 mm, slice gap = 1 mm, and matrix size = 96×96. For EPI, TR = 2000 ms, TE = 30 ms, flip angle = 90 degrees, and effective echo-spacing = 0.578 / 2 = 0.289 ms.
Results
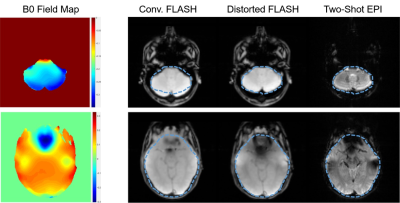
The estimated B0 field maps and effects of inverse distortion correction are shown in Fig 2 for two representative slices. After inverse distortion correction, the FLASH images became more consistent to EPI images.
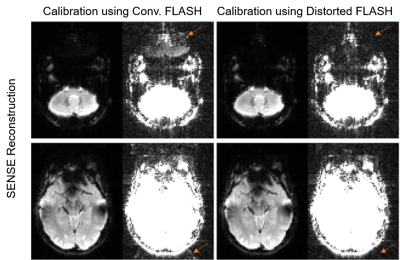
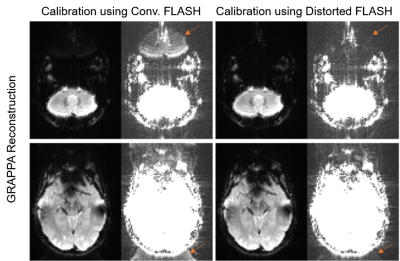
Figs 3 and 4 demonstrate the proposed method with SENSE and GRAPPA, respectively. Compared with conventional FLASH based calibration, the proposed method substantially reduced the residual aliasing artifact for both SENSE and GRAPPA.
Discussion and Conclusions
Our proposed method uses dual-echo FLASH with inverse distortion correction as the calibration data. It can effectively reduce the artifact related to the inconsistent geometry between FLASH and EPI images. This method can be particularly useful at higher fields where the field inhomogeneity is more pronounced. In practice, the scan time of dual-echo FLASH is very similar to conventional single-echo FLASH, which ranges from 5 to 20 seconds. Optionally, the derived B0 field maps can also be used to correct the distortion of EPI after parallel imaging reconstruction is finished. Compared to a recent study where dual echo FLASH is used for EPI reconstruction with iterative computation6, our approach requires less computation time and can be more easily combined any reconstruction algorithm. Future studies can compare the proposed method with more advanced EPI based calibration techniques, such as FLEET2, GESTE7, and VC-SAKE8.Acknowledgements
This work was supported by the Hong Kong Research Grant Council (Grants C7048-16G and HKU17103015 to E.X.W.).References
1. Talagala, S. L., Sarlls, J. E., Liu, S. & Inati, S. J. Improvement of temporal signal-to-noise ratio of GRAPPA accelerated echo planar imaging using a FLASH based calibration scan. Magn. Reson. Med. 75, 2362-2371, doi:10.1002/mrm.25846 (2016).
2. Polimeni, J. R. et al. Reducing sensitivity losses due to respiration and motion in accelerated echo planar imaging by reordering the autocalibration data acquisition. Magn. Reson. Med. 75, 665-679, doi:10.1002/mrm.25628 (2016).
3. Barth, M., Breuer, F., Koopmans, P. J., Norris, D. G. & Poser, B. A. Simultaneous multislice (SMS) imaging techniques. Magn. Reson. Med. 75, 63-81, doi:10.1002/mrm.25897 (2016).
4. Chiou, J. Y., Ahn, C. B., Muftuler, L. T. & Nalcioglu, O. A simple simultaneous geometric and intensity correction method for echo-planar imaging by EPI-based phase modulation. IEEE Trans. Med. Imaging 22, 200-205, doi:10.1109/TMI.2002.808362 (2003).
5. Xie, V. B., Lyu, M. & Wu, E. X. EPI Nyquist ghost and geometric distortion correction by two-frame phase labeling. Magn. Reson. Med. 77, 1749-1761, doi:10.1002/mrm.26251 (2017).
6. Yarach, U. et al. Model-based iterative reconstruction for single-shot EPI at 7T. Magn. Reson. Med., 10.1002/mrm.26633, doi:10.1002/mrm.26633 (2017).
7. Hoge, W. S., Tan, H. & Kraft, R. A. Robust EPI Nyquist ghost elimination via spatial and temporal encoding. Magn. Reson. Med. 64, 1781-1791, doi:10.1002/mrm.22564 (2010).
8. Lyu, M. et al. in Proceedings of the 25th Annual Meeting of ISMRM. 515.
Figures