3484
An integrative deep learning model to distinguish between normal and atherosclerotic carotid arteries on black-blood vessel wall MRI1Institute of Artificial Intelligence and Robotics, Xi’an Jiaotong University, Xi'an, China, 2Department of Radiology, University of Washington, Seattle, WA, United States, 3Philips Research North America, Cambridge, Cambridge, MA, United States
Synopsis
Vessel wall (VW) MRI has been used to characterize atherosclerotic plaques but the review process is complex. To facilitate the translation of VWMRI into clinical application, we utilized deep convolutional neural networks (CNN) to distinguish between normal and atherosclerotic carotid arteries automatically in black-blood (BB) VWMRI. Trained with a dataset that contains both normal and diseased carotid arteries with expert labeling, an integrative deep CNN model was developed and yielded better automatic diagnosis accuracy of carotid atherosclerosis (85.18%) compared with other existing methods. This model may be used as an initial screening to separate normal from diseased arteries.
Introduction
Direct plaque imaging with Vessel wall (VW) MRI allows for early detection of atherosclerotic changes in carotid arteries before luminal stenosis becomes apparent, providing valuable information for individual cardiovascular risk assessment and treatment planning.1,2 However, the translation of VWMRI from bench to bedside has been limited by the complicated image analysis process, presence of plaque-mimicking artifacts, and lack of experience among radiologists, resulting in only moderate inter-reader reproducibility.3 Identifying patients with atherosclerotic carotid arteries represents an important step in further characterization. In this study, we leverage recent advances in deep convolutional neural networks (CNN) to develop an automatic algorithm to differentiate atherosclerotic from normal carotid arteries on black-blood (BB) VW MRI.Methods
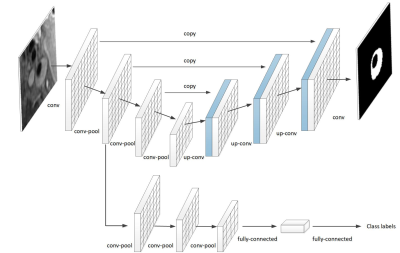
Model:Our goal was to distinguish between normal and diseased arteries. Our classification process relied on both morphology and patterns of carotid vessel wall and was implemented by conventional CNN with the aid of feedback from morphological information. This morphological information was extracted via a carotid vessel wall segmentation process and utilized for enhancing the classification. Based on the auxiliary morphological information, we developed an integrative CNN, in which a framework with two common layers was proposed to parallelize a classification task for diagnosis and a segmentation task for extracting morphological information. The segmentation branch of the proposed framework was to begin with U-net architecture,4 where a contracting path is constructed to capture context and a symmetric expansion path is built up to enable precise segmentation in a pixel-by-pixel manner. The number of layers in the U-net network and the kernels in each layer were reduced, i.e., 80*80 with consideration of the size of input images in our study. Most importantly, in order to predict a diagnosis from image to class label in a parallel manner with the existing segmentation branch, we added a branch composed of a traditional CNN and fully-connected neural network. The classification branch shares two common layers with the segmentation branch so that the morphological information from the segmentation branch can also be exploited by the classification branch. The proposed model architecture is illustrated in Figure 1.
Dataset:The dataset included 1072 carotid MRIs from a previous multicenter cohort study on patients with recent cerebrovascular events that may or may not be of carotid origin.5 Each scan included 16 axial slices of multiple contrast weightings. Trained readers analyzed all available slices by delineating lumen and outer wall boundaries and assigning a lesion type based on modified American Heart Association (AHA) criteria.6 As AHA Type I-II lesions are indistinguishable from normal vessel walls on MRI, and considered a reversible stage of atherosclerotic changes in histology,7 slices with AHA Type III-VIII lesions were defined as atherosclerotic in this study. Only the T1-weighted BB MRI sequences for each subject was used, and we randomly used 963 subjects for training and 107 subjects for testing. As a result, 16000 slices were included for training and 1375 slices were used for testing. Additionally, one tenth of training slices were randomly selected from the training set for validation during training.
Results
We
implemented the proposed model using multi-task training with a fixed coefficient
(classification loss weight: segmentation loss weight = 0.5:1), and only flip,
rotation and shift augmentation strategies were used for training. Diagnostic
prediction was evaluated with two metrics: accuracy and area under the receiver
operating characteristic curve (AUC), and the segmentation result was evaluated
with two metrics: pixel-wise accuracy and Dice’s coefficient indicating spatial
overlap between binary segmentation results and ground truth. Further the
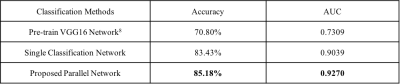
Pre-trained VGG16 network8 was implemented as the
baseline for classification. In addition, the proposed model was also compared
with a single classification network (classification loss weight: segmentation
loss weight = 1:0) without auxiliary morphological information. The
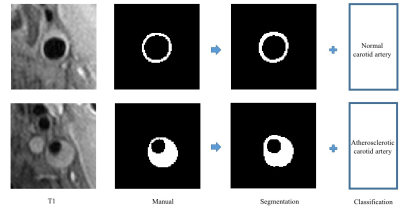
classification results are shown in Table 1, while examples of input images and
the results obtained from the proposed integrative CNN are presented in Figure
2, respectively. The proposed integrative CNN outperformed most other methods
in classification. Meanwhile, pixel-wise segmentation of carotid arteries
vessel walls achieved 97.39% (accuracy), 0.7090 (Dice) in the same test data
set.
Conclusion
In this study, by utilizing auxiliary
carotid artery morphological information, we combined the diagnosis of
atherosclerotic carotid arteries and carotid artery vessel wall segmentation using
deep CNN in a parallel manner, followed by a significant diagnosis performance
boost. Without the reviewer intervention required by the previous studies, the
proposed integrative CNN model can automatically screen atherosclerotic carotid
arteries from normal carotid arteries. This model may be used in screening
review to separate normal carotid from diseased arteries until the latter
undergo further analysis.
Acknowledgements
This study is supported by the National Institutes of Health (R01 HL103609, R01 NS083503) and the National Natural Science Foundation of China (81271536). The authors thank CARE-II study investigators and the support of NVIDIA Corporation with donation of the Titan Xp GPU. The authors also thank Philips Healthcare for assistance in training and implementation of MRI techniques at the participating hospitals.
References
1. Zavodni AE, Wasserman BA, McClelland RL, et al. Carotid artery plaque morphology and composition in relation to incident cardiovascular events: The Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2014; 271:381-389.
2. Sun J, Zhao XQ, Balu N, et al. Carotid Plaque Lipid Content and Fibrous Cap Status Predict Systemic CV Outcomes: The MRI Substudy in AIM-HIGH. JACC Cardiovasc Imaging 2017; 10:241-249.
3. Chu B, Phan BA, Balu N, Yuan C, Brown BG, Zhao XQ. Reproducibility of carotid atherosclerotic lesion type characterization using high resolution multicontrast weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2006; 8:793-799.
4. Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. Proc. 2015 Int. Conf. Medical Image Computing and Computer-Assisted Intervention. Springer, Cham, 2015; 234-241.
5. Zhao X, Hippe DS, Li R, et al. Prevalence and Characteristics of Carotid Artery High‐Risk Atherosclerotic Plaques in Chinese Patients with Cerebrovascular Symptoms: A Chinese Atherosclerosis Risk Evaluation II Study. Journal of the American Heart Association 2017;6(8): e005831.
6. Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002; 106:1368-1373.
7. Kolodgie FD, Burke AP, Nakazawa G, Virmani R. Is pathologic intimal thickening the key to understanding early plaque progression in human atherosclerotic disease? Arterioscl Throm Vas 2007; 27:986-989.
8. Simonyan K, Zisserman A. Very deep convolutional networks for large-scale image recognition. Computer Science 2014.