3479
“3D-Stars” Cine MRI for the Coronary Arteries: Initial Steps towards Volumetric Endothelial Function Assessment1Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 4Department of Radiology, University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 5Center for Biomedical Imaging (CIBM), Lausanne, Switzerland, 6Siemens Healthineers, Baltimore, MD, United States, 7Siemens Healthineers, Los Angeles, CA, United States
Synopsis
Recently, 2D coronary cine MRI has been shown to noninvasively assess coronary endothelial dysfunction, which is an early manifestation of atherosclerosis and a predictor of future acute events. However, atherosclerosis is a diffuse process, whereas this 2D approach provides local functional measures. Here, we present a free-breathing golden-angle 3D stack-of-stars cine sequence with isotropic spatial resolution combined with respiratory self-gating and 5D-GRASP reconstruction to image the proximal and mid segments of the right coronary artery. We call this new method “3D-Stars” and show feasibility to obtain volumetric cross-sectional area measures that can be used in future endothelial function studies.
Introduction
Abnormal coronary endothelial function (CEF) occurs early in atherosclerosis, is a marker for sub-clinical atherosclerotic disease, and independently predicts cardiovascular events1,2. However, CEF is rarely measured in clinical practice due to the invasive nature of conventional catheterization-based CEF measures. Non-invasive CEF measures3 were recently introduced with 2D spiral cine MRI and isometric handgrip exercise (IHE), an endothelial-dependent stressor4. In healthy subjects, cross-sectional area (CSA) increases with IHE but not in patients with coronary artery disease (CAD). MRI-based CEF measures were shown to be inversely related to underlying CAD, and reproducible3,5. Despite the significant advance that 2D CEF-MRI offers over conventional invasive methods2, several technical limitations prevent the widespread use of 2D CEF-MRI. First, atherosclerosis is a diffuse process, whereas each 2D-CEF-MR measure is derived locally from a single 8-mm coronary segment. Second, 2D CEF-MRI scans require 20-25s breath-holds which are difficult for some patients and limit the spatial resolution. Although sub-millimetric resolution is achieved in-plane, the slice thickness remains about 8 mm.3 To address these, we developed a respiratory resolved and self-gated, targeted 3D stack-of-stars cine method, called “3D-Stars” hereafter, to assess CEF in larger portions of the coronary tree during free-breathing and with isotropic resolution during the time of IHE (<6 min).Methods
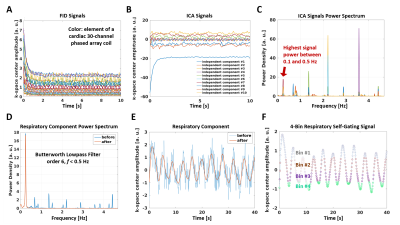
Acquisition: A targeted 3D-Stars volume was prescribed using an interactive real-time sequence6 to image the proximal and mid portions of right coronary artery (RCA). 3D-Stars uses a continuous segmented hybrid in-plane radial/through-plane Cartesian (stack-of-stars) acquisition. For each data segment, 20 radial lines are acquired along the slice direction with center-out ordering and same azimuthal angle, after chemical shift selective fat-saturation7. Consecutive segments are rotated by the golden angle8. The k-space center is sampled at the beginning of each data segment for offline respiratory self-gating9, 10 (see below). The novel prototype gradient-echo sequence was tested in 3 healthy volunteers in a 3-T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with a 30-channel coil array, 1.4-mm isotropic resolution, 300x300x28-mm3 field-of-view, 3000 lines/slice, and 5’21” acquisition time.Gating Signals: Independent component analysis11 was used to reduce k-space center amplitude signals from all coils (Figure 1A) to only 10 components (Figure 1B). Among these, a respiratory component was then automatically selected based on its frequency content (Figure 1C) and filtered (Figure 1D-E) to provide a self-gating signal9, 10 (Figure 1F). Peripheral pulse oximetry was used for cardiac gating.
Reconstruction: Motion-unresolved gridded12 data were displayed at the scanner. Offline, data were sorted in 15 cardiac and 4 respiratory phases, and reconstructed with a prototype 5D-GRASP13 algorithm to yield motion-resolved 3D cine images (MATLAB, BART14).
Analysis: For each dataset, diastolic volumes at end-expiration were selected for coronary reformatting15 and analysis. CSA measures were obtained with a custom-built software that performed center-line tracking16 and a full-width at half maximum 3D algorithm in adjacent 4-mm-long tubes along the center line.
Results
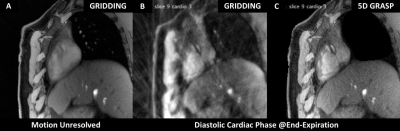
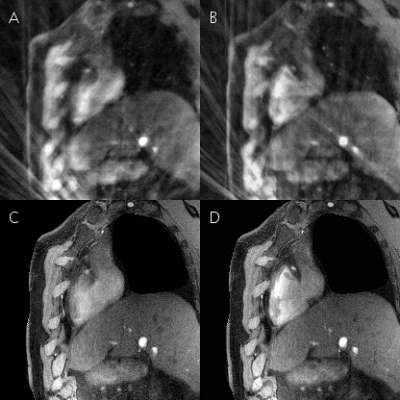
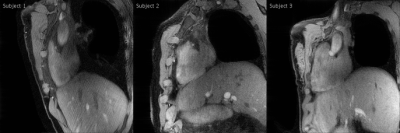
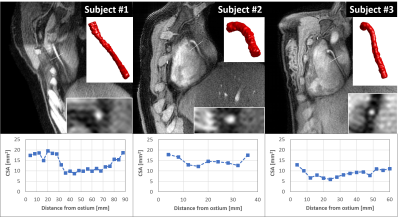
Gridded motion-unresolved data show blurring at the level of the heart (Figure 2A). After sorting into cardiac and respiratory phases, gridding reconstruction of a diastolic cine image at end-expiration shows heavy streaking artifacts (Figure 2B). Reduced artifacts and improved depiction of the coronary artery are obtained with the 5D-GRASP reconstruction (Figure 2C). Animated Figure 3 shows a selected slice from gridded and 5D-GRASP reconstructed images through cardiac and respiratory phases for one subject. Animated Figure 4 displays 5D-GRASP images selected from a diastolic phase at end-expiration. Multiplanar reformats from the same 5D-GRASP data show good depiction of proximal RCAs in all 3 subjects (Figure 5). Mean vessel length and CSA (along the vessel) in the 3 subjects were (µ±σ): 58.7±26.0 mm and 12.2±2.9 mm2, respectively.Discussion
The proposed free-breathing targeted 3D coronary cine technique shows promising image quality and feasibility for imaging the RCA in <6 minutes, while resolving cardiac and respiratory motion. Importantly, the isotropic and volumetric nature of the datasets allows for quantification of CSA along several centimeters of the RCA with a semiautomated tool in 3D. Future work is needed to optimize the spatial resolution, to test this approach in the left coronary artery, and to validate the new 3D-Stars CEF-MRI with established 2D CEF-MRI measures. 3D-Stars has the potential to provide the first approach for non-invasive and volumetric assessment of CEF and therefore, theoretically, a better characterization of the spatial heterogeneity of functional coronary atherosclerotic disease.Acknowledgements
Work supported by NIH HL120905, NIH HL125059, NIH HL61912, AHA 17SDG33671007 and the District of Columbia Women’s Board (DCWB).References
1. Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW and Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046-51.
2. Schachinger V, Britten MB and Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899-906.
3. Hays AG, Hirsch GA, Kelle S, Gerstenblith G, Weiss RG and Stuber M. Noninvasive visualization of coronary artery endothelial function in healthy subjects and in patients with coronary artery disease. J Am Coll Cardiol. 2010;56:1657-65.
4. Weiss RG, Bottomley PA, Hardy CJ and Gerstenblith G. Regional myocardial metabolism of high-energy phosphates during isometric exercise in patients with coronary artery disease. N Engl J Med. 1990;323:1593.
5. Hays AG, Iantorno M, Soleimanifard S, Steinberg A, Schär M, Gerstenblith G, Stuber M and Weiss RG. Coronary vasomotor responses to isometric handgrip exercise are primarily mediated by nitric oxide: a noninvasive MRI test of coronary endothelial function. Am J Physiol Heart Circ Physiol. 2015;308:H1343-50.
6. Uecker M, Zhang S, Voit D, Karaus A, Merboldt KD and Frahm J. Real-time MRI at a resolution of 20 ms. NMR Biomed. 2010;23:986-94.
7. Haase A, Frahm J, Hanicke W and Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Phys Med Biol. 1985;30:341-4.
8. Winkelmann S, Schaeffter T, Koehler T, Eggers H and Doessel O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging. 2007;26:68-76.
9. Bonanno G, Piccini D, Maréchal B, Zenge MO and Stuber M. A New Binning Approach for 3D Motion Corrected Self-Navigated Whole-Heart Coronary MRA Using Independent Component Analysis of Individual Coils. Paper presented at: Proc Intl Soc Magn Reson Med 2014; Milan, Italy.
10. Bonanno G, Hays AG, Weiss RG and Schär M. Self-Gated Golden Angle Spiral CINE MRI for Coronary Endothelial Function Assessment. Paper presented at: Proc Intl Soc Magn Reson Med 2017; Honolulu, HI.
11. Hyvarinen A and Oja E. Independent component analysis: algorithms and applications. Neural Netw. 2000;13:411-30.
12. Jackson JI, Meyer CH, Nishimura DG and Macovski A. Selection of a convolution function for Fourier inversion using gridding [computerised tomography application]. IEEE Trans Med Imaging. 1991;10:473-8.
13. Feng L, Axel L, Chandarana H, Block KT, Sodickson DK and Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med. 2016;75:775-88.
14. BART Toolbox for Computational Magnetic Resonance Imaging. DOI: 10.5281/zenodo.592960 [computer program].
15. Etienne A, Botnar RM, Van Muiswinkel AM, Boesiger P, Manning WJ and Stuber M. "Soap-Bubble" visualization and quantitative analysis of 3D coronary magnetic resonance angiograms. Magn Reson Med. 2002;48:658-66.
16. Soleimanifard S, Schar M, Hays AG, Weiss RG, Stuber M and Prince JL. Vessel Centerline Tracking and Boundary Segmentation in Coronary Mra with Minimal Manual Interaction. Proc IEEE Int Symp Biomed Imaging. 2012:1417-1420.
Figures